
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
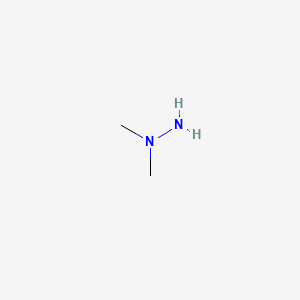

1. 1,1-dimethyhydrazine
2. Dimazine
3. Dimazine Dihydrochloride
4. Dimazine Hydrochloride
5. Unsymmetrical Dimethylhydrazine
1. 57-14-7
2. N,n-dimethylhydrazine
3. Dimazine
4. Gem-dimethylhydrazine
5. Unsymmetrical Dimethylhydrazine
6. 1,1-dimethylhydrazin
7. Udmh
8. Unsym-dimethylhydrazine
9. Hydrazine, 1,1-dimethyl-
10. U-dimethylhydrazine
11. 1,1-dimethyl Hydrazine
12. Uns-dimethylhydrazine
13. Dimazin
14. As-dimethylhydrazine
15. Hydrazine, N,n-dimethyl-
16. Asymmetric Dimethylhydrazine
17. Rcra Waste Number U098
18. Unsymmetrical-dimethylhydrazine
19. Dimethylhydrazine, Unsym.
20. Nsc 60517
21. 1.1-dimethylhydrazine
22. Niesymetryczna Dwu Metylohydrazyna
23. (ch3)2nnh2
24. 4wpq90n53j
25. Dimethylhydrazine, Unsymmetrical
26. Dtxsid1020516
27. Nsc-60517
28. As-dimethyl Hydrazine
29. Asymmetric-dimethylhydrazine
30. Ccris 258
31. Dimethylhydrazine Unsymmetrical
32. N,n-dimetilidrazina
33. N,n-dimetilidrazina [italian]
34. Hsdb 528
35. 1,1-dimethylhydrazin [german]
36. Einecs 200-316-0
37. Un1163
38. Dimethyl Hydrazine (dmh)
39. Rcra Waste No. U098
40. Brn 0605261
41. Unii-4wpq90n53j
42. Niesymetryczna Dwu Metylohydrazyna [polish]
43. 1,1-dimethyldiazane
44. Dimethylhydrazine, As
45. Asym-dimethylhydrazine
46. Hydrazine,n-dimethyl-
47. Hydrazine,1-dimethyl-
48. N,n-dimethyl Hydrazine
49. N,n-dimethyl-hydrazine
50. 1,1-dimethyl-hydrazine
51. Me2nnh2
52. N',n'-dimethylhydrazine
53. Dsstox_cid_516
54. Ec 200-316-0
55. Dsstox_rid_75637
56. Dsstox_gsid_20516
57. Unsymmetrical-dimethylhyrazine
58. N,n-dimethylhydrazine, 98%
59. Wln: Zn1&1
60. Chebi:18853
61. Nsc60517
62. 1,1-dimethylhydrazine [mi]
63. Tox21_302358
64. Mfcd00007628
65. Zinc59447665
66. 1,1-dimethylhydrazine [hsdb]
67. 1,1-dimethylhydrazine [iarc]
68. Akos000119664
69. Un 1163
70. Cas-57-14-7
71. Ncgc00255194-01
72. D0740
73. C19233
74. A831327
75. Q161296
76. Q-200052
77. Dimethylhydrazine, Unsymmetrical [un1163] [poison]
| Molecular Weight | 60.10 g/mol |
|---|---|
| Molecular Formula | C2H8N2 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 60.068748264 g/mol |
| Monoisotopic Mass | 60.068748264 g/mol |
| Topological Polar Surface Area | 29.3 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 11.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dimethylhydrazine administered by injection to a variety of species, including rat, rabbit, cat, dog, and monkey was rapidly absorbed into the blood and quite rapidly excreted via the kidneys. No preferential organ of storage was seen, and the urinary concentration was considered a more sensitive indication of exposure than blood levels. In rats given low doses (0.78 mg/kg), 30% of the injected radioactivity appeared as respiratory CO2 in 10 hr. Again, urine was the major excretory route. The urinary product was unchanged dimethylhydrazine. Other compounds identified in the urine of both rats and dogs following injections of dimethylhydrazine include a glucose hydrazine of dimethylhydrazine (3-10%) and an undetermined hydrazine (20-25%), and dimethylhydrazine accounted for 50-60%. Dogs and rats showed the same absorption and excretion patterns.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 1298
Dimethylhydrazine showed first order absorption processes when applied /dermally/. Subcutaneous administration resulted in much higher blood levels and almost complete absorption. In vitro application to rabbit skin showed evaporation of 85% of the dose, thus accounting for the low observed absorption. Elimination of dimethylhydrazine was rapid, and the terminal elimination half-life was 0.3-1.5 hours. From 3-19% of the dose was eliminated in urine.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 1299
Approximately 50% of the absorbed dose is excreted in 24 hr.
International Labour Office. Encyclopedia of Occupational Health and Safety. Vols. I&II. Geneva, Switzerland: International Labour Office, 1983., p. 1070
Unsymmetrical dimethylhydrazine is /biotransformed/ ...to carbon dioxide and unknown metabolites which are excreted with free... /1,1-dimethylhydrazine/ into the urine.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 140
For more Absorption, Distribution and Excretion (Complete) data for 1,1-DIMETHYLHYDRAZINE (8 total), please visit the HSDB record page.
Formaldehyde was formed by oxidative demethylation of 1,1-dimethylhydrazine by rat liver microsomes. Phenobarbital or 3-methylcholanthrene pretreatment enhanced demethylase activity.
PMID:4391185 Wittkop JA et al; Arch Biochem Biophys 134 (2): 308-15 (1969)
Rats admin low dose of (14)C 1,1-dimethylhydrazine metabolized approx 30% to (14)C labeled carbon dioxide in 10 hr. Conversion of convulsive dose to carbon dioxide amounted to slightly more than 13% at end of 20 hr. At least 50% of administered radioactivity appeared in urine in 2-day period.
Dost FN et al; Biochem Pharmacol 15 (9): 1325 (1966)
N-oxidation of alkylhydrazines was catalyzed by mouse liver microsomal mixed function oxidase. At pH 7.7 and 25 C, methylhydrazine and 1,1-dimethylhydrazine have nearly the same maximal n-oxidation rate as dimethylaniline.
PMID:4729304 Prough RA; Arch Biochem Biophys 158 (1): 442-4 (1973)
1,1-Dimethylhydrazine when added to suspension of rat liver microsomes exhibited binding spectra like those seen for nitrogenous ligands to cytochrome P450.
PMID:7391974 Hines RN, Prough RA; J Pharmacol Exp Ther 214 (1): 80-86 (1980)
For more Metabolism/Metabolites (Complete) data for 1,1-DIMETHYLHYDRAZINE (6 total), please visit the HSDB record page.
Elimination of dimethylhydrazine was rapid, and the terminal elimination half-life was 0.3-1.5 hours.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 1299
The precise mechanism of dimethylhydrazine toxicity is uncertain. In addition to the contact irritant effects, the acute effects of dimethylhydrazine exposure may involve the central nervous system as exemplified by tremors and convulsions and behavioral changes at sublethal doses. /It was/... noted that the deaths probably involve respiratory arrest and cardiovascular collapse. The central nervous system as a target is consistent with the delayed latency in response reported for dimethylhydrazine. There is some evidence that 1,1-dimethylhydrazine may act as an inhibitor of glutamic acid decarboxylase, thereby adversely affecting the aminobutyric acid shunt, and could explain the latency of CNS effects. Furthermore, vitamin B6 analogues that act as coenzymes in the aminobutyric acid shunt have been shown to be effective antagonists to 1,1-dimethylhydrazine toxicity.
CLS/NAS; Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 1; Dimethylhydrazine p.173 (2000)
ABOUT THIS PAGE
15
PharmaCompass offers a list of 1,1-Dimethylhydrazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right 1,1-Dimethylhydrazine manufacturer or 1,1-Dimethylhydrazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred 1,1-Dimethylhydrazine manufacturer or 1,1-Dimethylhydrazine supplier.
PharmaCompass also assists you with knowing the 1,1-Dimethylhydrazine API Price utilized in the formulation of products. 1,1-Dimethylhydrazine API Price is not always fixed or binding as the 1,1-Dimethylhydrazine Price is obtained through a variety of data sources. The 1,1-Dimethylhydrazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 1,1-Dimethylhydrazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 1,1-Dimethylhydrazine, including repackagers and relabelers. The FDA regulates 1,1-Dimethylhydrazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 1,1-Dimethylhydrazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A 1,1-Dimethylhydrazine supplier is an individual or a company that provides 1,1-Dimethylhydrazine active pharmaceutical ingredient (API) or 1,1-Dimethylhydrazine finished formulations upon request. The 1,1-Dimethylhydrazine suppliers may include 1,1-Dimethylhydrazine API manufacturers, exporters, distributors and traders.
1,1-Dimethylhydrazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 1,1-Dimethylhydrazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 1,1-Dimethylhydrazine GMP manufacturer or 1,1-Dimethylhydrazine GMP API supplier for your needs.
A 1,1-Dimethylhydrazine CoA (Certificate of Analysis) is a formal document that attests to 1,1-Dimethylhydrazine's compliance with 1,1-Dimethylhydrazine specifications and serves as a tool for batch-level quality control.
1,1-Dimethylhydrazine CoA mostly includes findings from lab analyses of a specific batch. For each 1,1-Dimethylhydrazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
1,1-Dimethylhydrazine may be tested according to a variety of international standards, such as European Pharmacopoeia (1,1-Dimethylhydrazine EP), 1,1-Dimethylhydrazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (1,1-Dimethylhydrazine USP).
