
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Australia

0
South Africa

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ai3 24916
2. Ai3-24916
3. Ai324916
4. Girostan
5. Nsc 6396
6. Nsc-6396
7. Nsc6396
8. Tespa
9. Tespamin
10. Thio Tepa
11. Thio-tepa
12. Thiophosphamide
13. Triethylenethiophosphoramide
14. Tris(1-aziridinyl)phosphine Sulfide
1. Thio-tepa
2. 52-24-4
3. Triethylenethiophosphoramide
4. Thioplex
5. Thiophosphamide
6. Tiofosfamid
7. Tiofosyl
8. Thiofozil
9. Tespamin
10. Girostan
11. Oncotepa
12. Tespamine
13. Tiofozil
14. Stepa
15. Tri(aziridin-1-yl)phosphine Sulfide
16. Oncotiotepa
17. Thiotef
18. Tifosyl
19. Oncothio-tepa
20. Tespa
21. Tio-tef
22. Thio-tep
23. Tspa
24. Thio-tepa S
25. Tepadina
26. Ledertepa
27. Thiotriethylenephosphoramide
28. Tris(1-aziridinyl)phosphine Sulfide
29. Tio Tef
30. 1,1',1''-phosphorothioyltriaziridine
31. Nsc-6396
32. N,n',n''-triethylenethiophosphoramide
33. Triethylene Thiophosphoramide
34. Triaziridinylphosphine Sulfide
35. Triethylenethiophosphorotriamide
36. Tris(ethylenimino)thiophosphate
37. Cbc 806495
38. Phosphorothioic Acid Triethylenetriamide
39. Phosphine Sulfide, Tris(1-aziridinyl)-
40. Tri(ethyleneimino)thiophosphoramide
41. Nci-c01649
42. Nsc 6396
43. Sk 6882
44. Tris(aziridinyl)phosphine Sulfide
45. Aziridine, 1,1',1''-phosphinothioylidynetris-
46. Tri-1-aziridinylphosphine Sulfide
47. Tris(1-aziridinyl)phosphine Sulphide
48. N,n',n''-triethylenethiophosphamide
49. N,n',n''-triethylenethiophosphortriamide
50. Nsc6396
51. 1,1',1''-phosphinothioylidynetrisaziridine
52. N,n',n''-triethylenephosphorothioic Triamide
53. N,n',n''-tri-1,2-ethanediylthiophosphoramide
54. Thiophosphoramide, N,n',n''-tri-1,2-ethanediyl-
55. N,n',n''-tri-1,2-ethanediylphosphorothioic Triamide
56. Mls001333083
57. Mls003389372
58. 905z5w3gkh
59. Chebi:9570
60. Phosphorothioic Triamide, N,n',n''-tri-1,2-ethanediyl-
61. Ai 3-24916
62. Phosphorothioic Triamide, N,n',n''-triethylene-
63. Tri(1-aziridinyl)phosphine Sulfide
64. Aziridine,1,1',1''-phosphinothioylidynetris-
65. Ncgc00095042-04
66. Ncgc00095042-06
67. Thiophosphamidum
68. Dsstox_cid_1339
69. Dsstox_rid_76093
70. Dsstox_gsid_21339
71. Thiotepum
72. Tiotepa
73. Thiotepum [inn-latin]
74. Tiotepa [inn-spanish]
75. Cas-52-24-4
76. Smr000058542
77. Ccris 586
78. Hsdb 3258
79. Ai3-24916
80. Sr-05000001855
81. Einecs 200-135-7
82. Brn 0145978
83. Unii-905z5w3gkh
84. Wr-45312
85. Thiotepa [usp:inn:ban:jan]
86. Tepadina (tn)
87. Thioplex (tn)
88. Mfcd00145452
89. Rethio (tn)
90. Thiotepa (thioplex)
91. Spectrum_001689
92. Wr 45312
93. Thiotepa [hsdb]
94. Thiotepa [iarc]
95. Thiotepa [inn]
96. Thiotepa [jan]
97. Thiophosphoramide, N,n',n''-triethylene-
98. Thiotepa [mi]
99. Thiotepa [vandf]
100. Spectrum2_001557
101. Spectrum3_001594
102. Spectrum4_000208
103. Spectrum5_001641
104. Thiotepa [mart.]
105. Tris(aziridin-1-yl)-thioxo-$l^{5}-phosphane
106. Chembl671
107. Thiotepa [usp-rs]
108. Thiotepa [who-dd]
109. Thioplex (tn) (immunex)
110. Tris(aziridinyl)-phosphine Sulfide (thio-tepa)
111. Schembl4760
112. Thiotepa (jan/usp/inn)
113. Thiotepa [ema Epar]
114. Thio-tepa, 98%, Solid
115. Bspbio_003188
116. Kbiogr_000815
117. Kbioss_002169
118. 4-20-00-00052 (beilstein Handbook Reference)
119. Mls001333084
120. Mls002207150
121. Mls006009972
122. Bidd:gt0127
123. Divk1c_000817
124. Spectrum1503324
125. Spbio_001434
126. Tris(aziridin-1-yl)-sulfanylidene-lambda5-phosphane
127. Thiotepa [orange Book]
128. Gtpl7622
129. Dtxsid0021339
130. Thiotepa [usp Monograph]
131. Hms502i19
132. Kbio1_000817
133. Kbio2_002169
134. Kbio2_004737
135. Kbio2_007305
136. Kbio3_002688
137. N,n''-triethylenethiophosphamide
138. Ninds_000817
139. Hms1922a14
140. Hms2093e05
141. Hms2232p17
142. Hms3372d10
143. N,n''-triethylenethiophosphoramide
144. Pharmakon1600-01503324
145. Albb-034802
146. Amy33389
147. Bcp04110
148. Zinc1530867
149. Tox21_111399
150. Tox21_400065
151. Bdbm50418086
152. Ccg-39776
153. Nsc758455
154. S1775
155. Akos005267118
156. Cs-3119
157. Db04572
158. Gc10080
159. Nsc-758455
160. 1,1''-phosphinothioylidynetrisaziridine
161. Idi1_000817
162. Thiophosphoramide,n',n'' -triethylene-
163. N,n',n'''-triethylenethiophosphhoramide
164. Ncgc00095042-01
165. Ncgc00095042-02
166. Ncgc00095042-03
167. Ncgc00095042-05
168. Ncgc00095042-07
169. Ncgc00095042-08
170. Ncgc00095042-09
171. Phosphorothioic Tri(ethyleneamide)
172. Tris(aziridin-1-yl)-
173. E?-phosphanethione
174. As-16885
175. Hy-17574
176. N,n''-triethylenephosphorothioic Triamide
177. Nci60_013117
178. Sbi-0051814.p002
179. Aziridine,1',1''-phosphinothioylidynetris-
180. N,n''-tri-1,2-ethanediylthiophosphoramide
181. Phosphorothioic Triamide,n',n''-triethylene
182. Thiophosphoramide, N,n',n'' -triethylene-
183. Tris(aziridin-1-yl)-lambda5-phosphanethione
184. Ft-0600281
185. Tris(aziridin-1-yl)-$l^{5}-phosphanethione
186. Tris(aziridin-1-yl)-sulfanylidenephosphorane
187. Wln: T3ntj Aps&- At3ntj&- At3ntj
188. 1-[di(1-aziridinyl)phosphorothioyl]aziridine #
189. C07641
190. D00583
191. Phosphorothioic Triamide, N,n',n''-triethylene
192. Thiophosphoramide,n',n''-tri-1,2-ethanediyl-
193. Ab00052346-06
194. Ab00052346_07
195. 145t452
196. A828998
197. N,n''-tri-1,2-ethanediylphosphorothioic Triamide
198. Q416507
199. Q-201826
200. Sr-05000001855-1
201. Sr-05000001855-5
202. 1,1',1''-phosphinothioylidynetris(aziridine)
203. Brd-k09631521-001-05-7
204. Phosphorothioic Triamide,n',n''-tri-1,2-ethanediyl-
205. Z2574360269
206. Thiotepa, United States Pharmacopeia (usp) Reference Standard
207. 1631739-26-8
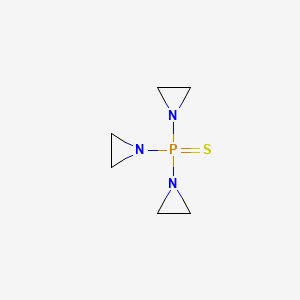
| Molecular Weight | 189.22 g/mol |
|---|---|
| Molecular Formula | C6H12N3PS |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 189.04895557 g/mol |
| Monoisotopic Mass | 189.04895557 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 194 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Thiotepa |
| PubMed Health | Thiotepa (Injection) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Thiotepa |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 15mg/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Thiotepa |
| PubMed Health | Thiotepa (Injection) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Thiotepa |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 15mg/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
Antineoplastic Agents, Alkylating; Myeloablative Agonists
National Library of Medicine's Medical Subject Headings. Thio-Tepa. Online file (MeSH, 2016). Available from, as of December 5, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Thio-tepa is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=THIO-TEPA&Search=Search
Thiotepa for Injection, USP has been tried with varying results in the palliation of a wide variety of neoplastic diseases. However, the most consistent results have been seen in the following tumors: 1. Adenocarcinoma of the breast. 2. Adenocarcinoma of the ovary. 3. For controlling intracavitary effusions secondary to diffuse or localized neoplastic diseases of various serosal cavities. 4. For the treatment of superficial papillary carcinoma of the urinary bladder. While now largely superseded by other treatments, thiotepa has been effective against other lymphomas, such as lymphosarcoma and Hodgkin's disease. /Included in US product label/
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b19b03db-471d-4ee7-b1b8-6eccc39c41b0
Thiotepa has been used as an ophthalmic instillation to prevent the recurrence of pterygium following surgical excision; however, postoperative beta-irradiation is generally preferred as preventive therapy because it results in a low incidence of recurrence and is relatively easy to administer. Many clinicians recommend that the use of thiotepa be limited to the management of pterygium which recurs following postoperative beta-irradiation. /NOT included in US product label/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
For more Therapeutic Uses (Complete) data for Thiotepa (6 total), please visit the HSDB record page.
Ophthalmic instillation of thiotepa may occasionally produce irritation or periorbital skin depigmentation; the depigmentation usually occurs 6 months or longer after cessation of treatment. Intrathecal administration of thiotepa has been associated with lower extremity weakness and pain and demyelination within the spinal cord in some patients; transient paresthesia of the lower extremities also has occurred following intrathecal administration of hypertonic solutions of the drug.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
Other reported adverse effects of thiotepa include pain at the injection site, headache, dizziness, blurred vision, conjunctivitis, dysuria, urinary retention, amenorrhea, and tightness of the throat. Some symptoms such as hyperuricemia or febrile reactions and exudation from subcutaneous lesions may be due to breakdown of tumor tissue. In some patients, intravesical administration of thiotepa has been reported to produce lower abdominal pain, vesical irritability, hematuria, and rarely hemorrhagic chemical cystitis.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
Hypersensitivity reactions, including allergic reactions, rash, urticaria, laryngeal edema, asthma, anaphylactic shock, and wheezing have occurred in patients receiving thiotepa. Contact dermatitis and alopecia also have been reported. Skin depigmentation has been reported following topical use of the drug.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
Nausea, vomiting, abdominal pain, and anorexia occur infrequently after administration of thiotepa. Stomatitis and ulceration of the intestinal mucosa also have been reported.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
For more Drug Warnings (Complete) data for Thiotepa (15 total), please visit the HSDB record page.
ThioTEPA is used a as conditioning treatment prior to allogeneic or autologous haematopoietic progenitor cell transplantation (HPCT) in haematological diseases in adult and paediatric patients. Also, when high dose chemotherapy with HPCT support it is appropriate for the treatment of solid tumours in adult and paediatric patients.
In combination with other chemotherapy medicinal products:
- with or without total body irradiation (TBI), as conditioning treatment prior to allogeneic or autologous haematopoietic progenitor cell transplantation (HPCT) in haematological diseases in adult and paediatric patients;
- when high dose chemotherapy with HPCT support is appropriate for the treatment of solid tumours in adult and paediatric patients. ". It is proposed that Tepadina must be prescribed by physicians experienced in conditioning treatment prior to haematopoietic progenitor cell transplantation.
The unstable nitrogen-carbon groups alkylate with DNA causing irrepairable DNA damage. They stop tumor growth by crosslinking guanine nucleobases in DNA double-helix strands, directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. These drugs act nonspecifically.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
Myeloablative Agonists
Agents that destroy bone marrow activity. They are used to prepare patients for BONE MARROW TRANSPLANTATION or STEM CELL TRANSPLANTATION. (See all compounds classified as Myeloablative Agonists.)
L01AC01
L01AC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AC - Ethylene imines
L01AC01 - Thiotepa
Route of Elimination
Urinary excretion of 14C-labeled thiotepa and metabolites in a 34-year old patient with metastatic carcinoma of the cecum who received a dose of 0.3 mg/kg intravenously was 63%.
Clearance
446 +/- 63 mL/min [female patients (45 to 84 years) with advanced stage ovarian cancer receiving 60 mg and 80 mg thiotepa by intravenous infusion on subsequent courses given at 4-week intervals]
In man, 50% of injection of (14)C-thiotepa either iv or locally into tumor was excreted in the first 6 hr and by 48 hr only low levels persisted. Absorption of orally administered dose of (14)C-thiotepa was variable: less than 1% of injected dose was recovered as unchanged drug in the urine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 89 (1975)
... Five min after intravenous or intraarterial injection of labelled thiotepa in Sprague-Dawley rats, slightly higher levels of radioactivity were found in plasma, heart, kidneys and lungs, compared to other organs; 94-98% of radioactivity administered intravenously was excreted in urine within 8.5 hr. Most of the urinary radioactivity was associated with unchanged thiotepa; tris(l-azridinyl)phosphine oxide (tepa) was responsible for about 30% of the radioactivity.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 127 (1990)
One hour after intraperitoneal injection of thiotepa at 9.3 mg/kg bw into Sprague-Dawley rats, radioactivity was found in plasma (5.4%), peritoneal fluid (26%), urine (1.9%), kidney (0.7%), liver (3.8%), lung (0.6%) and muscle (25.9%).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 127 (1990)
In patients with normal renal function, thiotepa, triethylenephosphoramide (TEPA), and unidentified metabolites with alkylating activity are excreted in urine. Urinary excretion of thiotepa, TEPA, and unidentified metabolites with alkylating activity is about 0.1-1.5, 4, and 13-24% of the dose, respectively, within the first 24-48 hours; urinary excretion of the parent drug is complete within the first 6-8 hours. Fecal excretion of the drug and its metabolites has not been studied. Following IV administration of high doses of thiotepa, the drug apparently is excreted in sweat to an appreciable extent.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
For more Absorption, Distribution and Excretion (Complete) data for Thiotepa (14 total), please visit the HSDB record page.
The major urinary metabolite in rats, rabbits and dogs following a single intravenous injection of (32)P-thiotepa was tepa, which is also an alkylating agent. Most of the radioactivity in mouse urine, however, was rccovered as inorganic phosphate.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 128 (1990)
... Five min after intravenous or intraarterial injection of labelled thiotepa in Sprague-Dawley rats, slightly higher levels of radioactivity were found in plasma, heart, kidneys and lungs, compared to other organs; 94-98% of radioactivity administered intravenously was excreted in urine within 8.5 hr. Most of the urinary radioactivity was associated with unchanged thiotepa; tris(l-azridinyl)phosphine oxide (tepa) was responsible for about 30% of the radioactivity.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 127 (1990)
In mice, thiotepa is rapidly metabolized to tris (1-aziridinyl)phosphine oxide (tepa): with 30 minutes only tepa and inorganic phosphate were detected in the urine and plasma. The mouse is exceptional in its ability to degrade the drug completely to inorganic phosphate.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 89 (1975)
Thiotepa appears to be extensively metabolized in the liver by the cytochrome P-450 microsomal enzyme system, principally via oxidative desulfuration to a triethylenephosphoramide (TEPA). Although TEPA is the only metabolite detected and identified in plasma, there is evidence that other unidentified metabolites also are formed. Following rapid IV injection of thiotepa in adults with normal renal and hepatic function, plasma concentrations of the drug appear to decline in a biphasic manner with a half-life of approximately 6-12 minutes in the initial phase and 1.2-2.9 hours in the terminal phase. The plasma elimination half-life of TEPA is about 10-21 hours.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
The urinary excretion of N,N',N"-triethylenethiophosphoramide (thioTEPA), and its metabolites N,N',N"-triethylenephosphoramide (TEPA), N,N'-diethylene,N"-2-chloroethylphosphoramide (monochloroTEPA) and thioTEPA--mercapturate was determined in patients receiving thioTEPA as part of a high-dose combination chemotherapy regimen with cyclophosphamide and carboplatin. The thioTEPA dose was 40 or 60 mg/sq m in short infusions, twice daily, during 4 days. Urine samples were collected after each voiding on each day of drug administration until 24-48 h after the last thioTEPA infusion. ThioTEPA, TEPA and monochloroTEPA concentrations were determined with gas chromatography and thioTEPA--mercapturate with liquid chromatography-mass spectrometry with direct sample injection. ThioTEPA was present in urine 30 min after infusion and was still excreted 18 hr after the last infusion. All metabolites were detected in urine 1 hr after infusion. Patients with a creatinine clearance above 140 ml/minl showed higher excretion of TEPA than patients with a creatinine clearance below 140 mL/min (12.8 versus 4.9%, p=0.01). The excretion of monochloroTEPA relative to the excreted amount of TEPA increased at lower pH values of the urine. The excretion of thioTEPA--mercapturate relative to the dose was higher in patients treated with 60 mg/sq m. Excretion of thioTEPA and monochloroTEPA both accounted for only 0.5% of the dose, while TEPA and thioTEPA--mercapturate both accounted for 11.1%.
PMID:11459998 van Maanen MJ et al; Anticancer Drugs 12 (6): 519-24 (2001)
1.5 to 4.1 hours
A total of 15 patients with residual ovarian cancer confined to the peritoneal cavity after first-line systemic chemotherapy were treated with thio-tepa in a phase I study. A total of 50 courses of thio-tepa were given ip in doses ranging from 30 to 80 mg/sq m. ... Peritoneal fluid concentrations declined rapidly in a first-order fashion, with a half-life of 0.96 + or - 0.1 hr. ...
PMID:1695127 Lewis C et al; Cancer Chemother Pharmacol 26 (4): 283-7 (1990)
A biexponential decline in thiotepa concentration in plasma was seen during the first hours after intravenous injection of thiotepa at 5 mg/kg bw in Swiss-Webster mice. The half-time was 0.21 min for the first phase and 9.62 min for the second.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V50 127-8 (1990)
Following rapid IV injection of thiotepa in adults with normal renal and hepatic function, plasma concentrations of the drug appear to decline in a biphasic manner with a half-life of approximately 6-12 minutes in the initial phase and 1.2-2.9 hours in the terminal phase. The plasma elimination half-life of triethylenephosphoramide (TEPA) is about 10-21 hours.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 426
The alkyl group is attached to the guanine base of DNA, at the number 7 nitrogen atom of the imidazole ring. They stop tumor growth by crosslinking guanine nucleobases in DNA double-helix strands, directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. These drugs act nonspecifically.
Thiotepa is a cytotoxic agent of the polyfunctional type, related chemically and pharmacologically to nitrogen mustard. The radiomimetic action of thiotepa is believed to occur through the release of ethylenimine radicals which, like irradiation, disrupt the bonds of DNA. One of the principal bond disruptions is initiated by alkylation of guanine at the N-7 position, which severs the linkage between the purine base and the sugar and liberates alkylated guanines.
NIH; DailyMed. Current Medication Information for Thiotepa (Thiotepa Injection, Powder, Lyophilized, for Solution (Updated: October 2015). Available from, as of March 31, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b19b03db-471d-4ee7-b1b8-6eccc39c41b0
The megakaryocytopoiesis in rats was studied following a single dose of thio-tepa to determine the mechanisms for the thrombocytopenia and subsequent recovery. The blood platelet number, platelet production (measured by (35)S incorporation into platelets), mean platelet volume, and the number and DNA content of bone marrow megakaryocytes were observed. The blood platelet counts, platelet production, and total megakaryocytes number decreased to low values following the administration of thio-tepa, and stayed low until regeneration started around day 10. The mean platelet volume increased from the normal 6.6 fL to 7.8 fL during the early regeneration days 8-14. The megakaryocytes were divided into four ploidy classes: 2N-4N, 8N, 16N, and 32N-64N. The number of megakaryocytes within all ploidy classes decreased to nearly zero within four days after the injection of thio-tepa. The regeneratin started around day 10 with increasing numbers of 2N-4N and 8N megakaryocytes, while the number of 16N and 32N-64N megakaryocytes increased more than four days later. It is concluded that decreased or blocked influx or progenitor cells into the megakaryocytes compartment is the main reason for thrombocytopenia after exposure to thio-tepa.
PMID:3081360 Tanum G; Exp Hematol 14 (3): 202-6 (1986)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

CAS Number : 52-24-4
Quantity Per Vial : 500
Sale Unit : mg
Price : $245.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1664000 / R11380

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?