
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
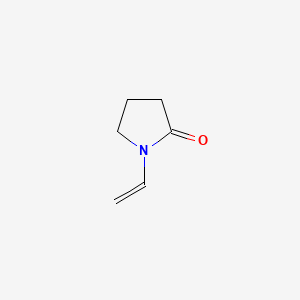

1. 1-vinyl-2-pyrrolidone
2. N- Vinyl Pyrrolidone
3. N-vinyl-2-pyrrolidinone
4. N-vinylpyrrolidone
1. 88-12-0
2. 1-vinylpyrrolidin-2-one
3. N-vinylpyrrolidone
4. 1-vinyl-2-pyrrolidone
5. N-vinyl-2-pyrrolidinone
6. Polyvinylpyrrolidone
7. Vinylpyrrolidone
8. 9003-39-8
9. 1-vinyl-2-pyrrolidinone
10. Povidone
11. 2-pyrrolidinone, 1-ethenyl-
12. 1-ethenylpyrrolidin-2-one
13. N-vinylpyrrolidinone
14. Polyvidone
15. 1-vinylpyrrolidone
16. Vinylbutyrolactam
17. Plasdone
18. Vinylpyrrolidinone
19. V-pyrol
20. Luviskol
21. 1-vinylpyrrolidinone
22. Vinyl-2-pyrrolidone
23. 25249-54-1
24. Polyclar At
25. N-vinyl Pyrrolidone
26. 1-ethenyl-2-pyrrolidinone
27. N-vinylpyrrolidone-2
28. 2-pyrrolidinone, 1-vinyl-
29. Poly(n-vinylpyrrolidone)
30. 1-vinyl-2-pyrrolidinone, Monomer
31. Poly(1-vinyl-2-pyrrolidone)
32. Pvp 40
33. Pvp
34. Nsc 10222
35. Mpk 90
36. 143 Rp
37. At 717
38. 1-vinyl-pyrrolidin-2-one
39. K 15
40. K 90
41. Pvp-40
42. Dtxsid2021440
43. Chebi:82551
44. Poly[1-(2-oxo-1-pyrrolidinyl)-1,2-ethanediyl]
45. Mfcd00003197
46. Nsc-10222
47. 2-pyrrolidinone, 1-ethenyl-, Trimer
48. 2-pyrrolidinone, Polymers
49. 76h9g81541
50. Dsstox_cid_1440
51. Dsstox_rid_76160
52. Dsstox_gsid_21440
53. Wln: /t5nvtj Ay*1*/
54. Mfcd01076626
55. Cas-88-12-0
56. Polyvinylpyrrolidine
57. 109412-11-5
58. K 25
59. K 115
60. Hsdb 7231
61. Einecs 201-800-4
62. Polyvinylpyrrolidone K 90
63. Polyvinylpyrrolidone K-30
64. 2-pyrrolidinone, Polymers, Compd. With Aluminum Acetate
65. Brn 0110513
66. Ccris 8581
67. Povidonepvp
68. Vinyl Pyrrolidone
69. Polyvinylpyrrolidon
70. Poly[1-(2-oxo-1-pyrrolidinyl)-1, .alpha.-hydro-.omega.-[[4-(iodo-131i)phenyl]methyl]-
71. Unii-76h9g81541
72. N-vinyl-pyrrolidone
73. N -vinylpyrrolidinone
74. 1-vinyl-2-pyrrolidon
75. Povidone Monomer
76. Vinylbutylolactam
77. N-vinylpyrrolidin-2-one
78. N-vinyl Pyrrolidin-2-one
79. N-vinyl-pyrrolidin-2-one
80. Pvp K3o
81. Crospovidone ~40,000
82. Ec 201-800-4
83. Poly (n-vinyl Pyrrolidone)
84. Schembl10869
85. Wln: T5nvtj A1u1
86. Pvp K15
87. Pvp K30
88. Pvp-k30
89. Polyvinylpyrrolidone Pvp K30
90. Povidone Monomer [mi]
91. Vinyl Pyrrolidone (vp)
92. Poly(1-vinylpyrrolidin-2-one)
93. Chembl1878943
94. Pvp - K-30 (pharm Grade)
95. Polyvinylpyrrolidone, Cross Linked
96. N-vinyl Pyrrolidone [inci]
97. 1-vinyl-2-pyrrolidone(stabilized With 200ppm Ammonium Hydroxide)
98. Nsc10222
99. Zinc3590964
100. Polyvinylpyrrolidone, M.w. 8,000
101. Tox21_202462
102. Tox21_300073
103. Nsc114022
104. Nsc142693
105. Nsc683040
106. N-vinyl-2-pyrrolidone, Optical Grade
107. Polyvinylpyrrolidone (mw ~40,000)
108. Polyvinylpyrrolidone, M.w. 10.000
109. Polyvinylpyrrolidone, M.w. 40.000
110. Polyvinylpyrrolidone, M.w. 58,000
111. Akos000119985
112. N-vinyl-2-pyrrolidone [iarc]
113. At18510
114. Cs-w020981
115. Fg-0420
116. Nsc-114022
117. Nsc-142693
118. Nsc-683040
119. Polyvinylpyrrolidone, M.w. 360.000
120. Ncgc00166252-01
121. Ncgc00166252-02
122. Ncgc00166252-03
123. Ncgc00254200-01
124. Ncgc00260011-01
125. Polyvinylpyrrolidone, M.w. 1,300,000
126. 2-pyrrolidinone, 1-ethenyl-[hsdb]
127. Ft-0608329
128. Ft-0645144
129. Ft-0655284
130. V0026
131. C19548
132. A817742
133. A843417
134. Poly(n-vinyl-2-pyrrolidone) (low M.wt.)
135. Q420628
136. Sr-01000944531
137. J-015891
138. Sr-01000944531-1
139. W-100417
140. 1-vinyl-2-pyrrolidinone, Saj First Grade, >=99.0%
141. F8881-5579
142. 3-chloro-5,6-difluoro-1-benzothiophene-2-carbonylchloride
143. 1-vinyl-2-pyrrolidinone, Contains Sodium Hydroxide As Inhibitor, >=99%
144. 1-vinyl-2-pyrrolidinone, Pharmaceutical Secondary Standard; Certified Reference Material
145. 1-vinyl-2-pyrrolidone (stabilized With N,n'-di-sec-butyl-p-phenylenediamine)
| Molecular Weight | 111.14 g/mol |
|---|---|
| Molecular Formula | C6H9NO |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 111.068413911 g/mol |
| Monoisotopic Mass | 111.068413911 g/mol |
| Topological Polar Surface Area | 20.3 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Pharmaceutic Aids; Plasma Substitutes
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
CLINICAL INDICATIONS: ...FOR ALL PATHOLOGICAL CONDITIONS WHERE THERE IS DECR IN MASS OF LIQ BLOOD: SHOCK STATE... HEMORRHAGE...SERIOUS BURNS. ANTHREPSIA & NEURO-TOXICOSES OF INFANTS.
Lefaux, R. Practical Toxicology of Plastics. Cleveland: CRC Press Inc., 1968., p. 292
...HAS BEEN USED IN A...SERIES OF PATIENTS TO REPLACE THE AQ HUMOR AFTER EXTRACTION OF CATARACTS, CORNEAL TRANSPLANTS, & ANTIGLAUCOMA OPERATIONS.
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 758
1. 1= PRACTICALLY NONTOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) ABOVE 15 G/KG, MORE THAN 1 QUART (2.2 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-245
When in complex with iodine, indicated for inducing antisepsis for prevention of infection in minor cuts, scrapes, and burns.
When in complex with iodine, indicated for inducing antisepsis for prevention of infection in minor cuts, scrapes, and burns.
Povidone itself has no microbicidal activity. [DB06812] exhibits rapid, potent, broad-spectrum antimicrobial properties. The clinical effectiveness of povidon-iodine on wound healing remains somewhat controversial; in few clinical studies investigating the effects of povidone-iodine on wound healing, topical administration of the complex was associated with no significant infections, but slower healing and mild to moderate discomfort on application.
Povidone itself has no microbicidal activity. [Povidone-iodine] exhibits rapid, potent, broad-spectrum antimicrobial properties. The clinical effectiveness of povidon-iodine on wound healing remains somewhat controversial; in few clinical studies investigating the effects of povidone-iodine on wound healing, topical administration of the complex was associated with no significant infections, but slower healing and mild to moderate discomfort on application.
Biocompatible Materials
Synthetic or natural materials, other than DRUGS, that are used to replace or repair any body TISSUES or bodily function. (See all compounds classified as Biocompatible Materials.)
Absorption
This pharmacokinetic data does not apply to povidone.
Route of Elimination
This pharmacokinetic data does not apply to povidone.
Volume of Distribution
This pharmacokinetic data does not apply to povidone.
Clearance
This pharmacokinetic data does not apply to povidone.
Absorption
This pharmacokinetic data does not apply to povidone.
Route of Elimination
This pharmacokinetic data does not apply to povidone.
Volume of Distribution
This pharmacokinetic data does not apply to povidone.
Clearance
This pharmacokinetic data does not apply to povidone.
WHEN GIVEN PARENTERALLY, UNEXCRETED PARTICLES ARE PHAGOCYTIZED BY CELLS OF RETICULOENDOTHELIAL SYSTEM & DEPOSITED IN STORAGE SITES IN LIVER, SPLEEN, LUNG, BONE MARROW...
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-245
...30-40% OF SUBTOSAN PASSES INTO THE URINE IN THE FIRST 24 HR & ABOUT 50% IN THE FIRST 2 DAYS. ...SMALLEST MOLECULES ARE...FIRST & MOST RAPIDLY EXCRETED. IT TAKES 10 DAYS AFTER PERFUSION FOR 80% OF AMT INJECTED TO BE ELIMINATED.
Lefaux, R. Practical Toxicology of Plastics. Cleveland: CRC Press Inc., 1968., p. 292
INTERMEDIATE MOLECULR WT PARTICLES MAY BE SLOWLY EXCRETED OVER SEVERAL MONTHS TO A YR.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-245
IN GROUPS OF RATS FED DIETS CONTAINING 1 & 10% PVP (MOLECULAR WEIGHT 38,000) FOR 2 YEARS...THERE WAS NO EVIDENCE THAT PVP WAS ABSORBED FROM THE INTESTINAL TRACT.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 471
For more Absorption, Distribution and Excretion (Complete) data for POLYVINYLPYRROLIDONE (6 total), please visit the HSDB record page.
This pharmacokinetic data does not apply to povidone.
This pharmacokinetic data does not apply to povidone.
PVP LABELLED WITH (14)C OR (131)I WAS NOT METABOLIZED TO ANY SIGNIFICANT DEGREE BY RATS, RABBITS OR DOGS FOLLOWING ITS IV INJECTION.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 472
This pharmacokinetic data does not apply to povidone.
This pharmacokinetic data does not apply to povidone.
Povidone-iodine is a water-soluble complex that mediates a bactericidal or virucidal action following the gradual liberation of free iodine from the complex at the application site to react with the pathogen. Please refer to the drug entry for [DB06812] for the full mechanism of action of the complex.
Povidone-iodine is a water-soluble complex that mediates a bactericidal or virucidal action following the gradual liberation of free iodine from the complex at the application site to react with the pathogen. Please refer to the drug entry for [Povidone-iodine] for the full mechanism of action of the complex.
ONE OF MAJOR PROPERTIES OF PVP IS ITS PHYSIO-CHEMICAL BEHAVIOR, WHICH IS... COMPARABLE TO THAT OF SERUM ALBUMEN... THUS IT FIXES & TRANSPORTS WATER AS WELL AS THE PRODUCTS FIXED BY SERUM ALBUMEN... IT CAN ALSO CARRY SUCH SUBSTANCES AS UREA, CREATININE, LACTOFLAVIN, COLORING MATTERS, HORMONES, & GLUCOSE.
Lefaux, R. Practical Toxicology of Plastics. Cleveland: CRC Press Inc., 1968., p. 290
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
89
PharmaCompass offers a list of N-Vinyl-2-Pyrrolidone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right N-Vinyl-2-Pyrrolidone manufacturer or N-Vinyl-2-Pyrrolidone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred N-Vinyl-2-Pyrrolidone manufacturer or N-Vinyl-2-Pyrrolidone supplier.
PharmaCompass also assists you with knowing the N-Vinyl-2-Pyrrolidone API Price utilized in the formulation of products. N-Vinyl-2-Pyrrolidone API Price is not always fixed or binding as the N-Vinyl-2-Pyrrolidone Price is obtained through a variety of data sources. The N-Vinyl-2-Pyrrolidone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 1-ethenylpyrrolidin-2-one manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 1-ethenylpyrrolidin-2-one, including repackagers and relabelers. The FDA regulates 1-ethenylpyrrolidin-2-one manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 1-ethenylpyrrolidin-2-one API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A 1-ethenylpyrrolidin-2-one supplier is an individual or a company that provides 1-ethenylpyrrolidin-2-one active pharmaceutical ingredient (API) or 1-ethenylpyrrolidin-2-one finished formulations upon request. The 1-ethenylpyrrolidin-2-one suppliers may include 1-ethenylpyrrolidin-2-one API manufacturers, exporters, distributors and traders.
1-ethenylpyrrolidin-2-one Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 1-ethenylpyrrolidin-2-one GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 1-ethenylpyrrolidin-2-one GMP manufacturer or 1-ethenylpyrrolidin-2-one GMP API supplier for your needs.
A 1-ethenylpyrrolidin-2-one CoA (Certificate of Analysis) is a formal document that attests to 1-ethenylpyrrolidin-2-one's compliance with 1-ethenylpyrrolidin-2-one specifications and serves as a tool for batch-level quality control.
1-ethenylpyrrolidin-2-one CoA mostly includes findings from lab analyses of a specific batch. For each 1-ethenylpyrrolidin-2-one CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
1-ethenylpyrrolidin-2-one may be tested according to a variety of international standards, such as European Pharmacopoeia (1-ethenylpyrrolidin-2-one EP), 1-ethenylpyrrolidin-2-one JP (Japanese Pharmacopeia) and the US Pharmacopoeia (1-ethenylpyrrolidin-2-one USP).
