
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
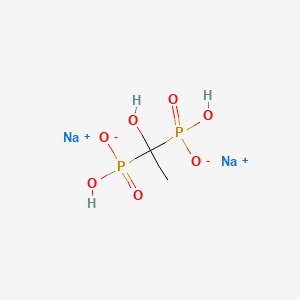

1. (1-hydroxyethylene)diphosphonic Acid
2. (1-hydroxyethylene)diphosphonic Acid, Tetrapotassium Salt
3. 1 Hydroxyethane 1,1 Diphosphonate
4. 1 Hydroxyethylidene 1,1 Bisphosphonate
5. 1,1 Hydroxyethylenediphosphonate
6. 1,1-hydroxyethylenediphosphonate
7. 1-hydroxyethane-1,1-diphosphonate
8. 1-hydroxyethylene Diphosphonate, Disodium
9. 1-hydroxyethylidene-1,1-bisphosphonate
10. Dicalcium Ehdp
11. Dicalcium Etidronate
12. Didronel
13. Diphosphonate, Disodium 1-hydroxyethylene
14. Diphosphonic Acid, Hydroxyethylidene
15. Disodium 1 Hydroxyethylene Diphosphonate
16. Disodium 1-hydroxyethylene Diphosphonate
17. Disodium Etidronate
18. Ehdp
19. Ehdp, Dicalcium
20. Ethanehydroxydiphosphonate
21. Ethanehydroxyphosphate
22. Etidronate
23. Etidronate, Dicalcium
24. Etidronate, Disodium
25. Etidronate, Sodium
26. Etidronate, Tetrapotassium Salt
27. Etidronic Acid
28. Hedp
29. Hedspa
30. Hydroxyethanediphosphonate
31. Hydroxyethylidene Diphosphonic Acid
32. Phosphonic Acid, (1-hydroxyethylidene)bis-, Disodium Salt
33. Salt Etidronate, Tetrapotassium
34. Sodium Etidronate
35. Tetrapotassium Salt Etidronate
36. Xidifon
37. Xidiphon
38. Xydiphone
1. 7414-83-7
2. Didronel
3. Disodium Etidronate
4. Sodium Etidronate
5. Sodium Ethidronate
6. Sodium Ethydronate
7. Disodium Ethydronate
8. Turpinal 2nz
9. Disodium Ethanol-1,1-diphosphonate
10. Etidronic Acid Disodium Salt
11. Etidronatedisodium
12. Disodium (1-hydroxyethylidene)diphosphonate
13. Phosphonic Acid, (1-hydroxyethylidene)bis-, Disodium Salt
14. Disodium 1-hydroxyethylidene Phosphonate
15. Chebi:4906
16. Hedpa;hedp
17. Disodium Dihydrogen (1-hydroxyethylidene)diphosphonate
18. Disodium Ethane-1-hydroxy-1,1-diphosphonate
19. (1-hydroxyethylidene)diphosphonic Acid, Disodium Salt
20. Etidronic Acid, Disodium Salt
21. 1-hydroxyethylidene-1,1-diphosphonic Acid Disodium Salt
22. (1-hydroxyethane-1,1-diyl)diphosphonic Acid Disodium Salt
23. M16pxg993g
24. Didronel R
25. 1-hydroxyethane-1,1-diphosphonic Acid Disodium Salt
26. Nsc-759157
27. Cas-7414-83-7
28. Dsstox_cid_9671
29. Disodium;hydroxy-[1-hydroxy-1-[hydroxy(oxido)phosphoryl]ethyl]phosphinate
30. Dsstox_rid_78804
31. Dsstox_gsid_29671
32. Disodium (1-hydroxyethane-1,1-diyl)bis[hydrogen (phosphonate)]
33. Etidronsaeure Dinatriumsalz
34. Etidronate Disodium Hydrate
35. Etidronic Acid Disodium
36. Ncgc00159352-02
37. 29329-71-3
38. Einecs 231-025-7
39. Sm-5600
40. Unii-m16pxg993g
41. Didronel Iv
42. Didronel Pmo
43. Phosphonic Acid, (1-hydroxyethylidene)bis-, Sodium Salt
44. Didronel (tn)
45. Ethane-1-hydroxy-1,1-diphosphonate, Disodium Salt
46. Etidronate Disodium [usan:usp:jan]
47. Mfcd00152567
48. Prestwick_1061
49. Disodium Dihydrogen (1-hydroxyethylidene)bisphosphonate
50. Ethane-1-hydroxy-1,1-diphosphonic Acid, Disodium Salt
51. Phosphonic Acid, (1-hydroxyethylidene)di-, Disodium Salt
52. Disodium Hydroxy-[1-hydroxy-1-[hydroxy(oxido)phosphoryl]ethyl]phosphinate
53. Chembl1201042
54. Dtxsid1029671
55. Etidronate Disodium [jan]
56. Etidronate Disodium (jp17/usp)
57. Disodium Etidronate [inci]
58. Etidronate Disodium [usan]
59. Hms1570n07
60. Hms2093p03
61. Hms2097n07
62. Etidronate Disodium [vandf]
63. Bcp22743
64. Etidronate Disodium [mart.]
65. Tox21_111596
66. Bdbm50247999
67. Etidronate Disodium [usp-rs]
68. Etidronate Disodium [who-dd]
69. Phosphonic Acid, P,p'-(1-hydroxyethylidene)bis-, Sodium Salt (1:2)
70. Akos015961949
71. Tox21_111596_1
72. Ac-2078
73. Ccg-213569
74. Ks-1297
75. Nsc 759157
76. Etidronate Disodium [orange Book]
77. Ncgc00017072-01
78. Ncgc00179380-04
79. Be164429
80. Etidronate Disodium [ep Monograph]
81. Etidronic Acid Disodium Salt [mi]
82. Etidronate Disodium [usp Monograph]
83. Disodium 1-hydroxyethane-1,1-diphosphonate
84. D4159
85. Ft-0630369
86. D00314
87. H11387
88. Etidronate Disodium;hedpa Disodium;hedp Disodium
89. A838033
90. Etidronate Disodium Hydrate, >=97% (nmr), Solid
91. W-104431
92. 1-hydroxyethane 1,1-diphosphonic Acid Disodium Salt
93. Disodium Etidronate;sm-5600; Sm-5600; Sm-5600
94. Q27106537
95. Sodium 1-hydroxyethane-1,1-diylbis(hydrogenphosphonate)
96. Disodium [1-[oxidanidyl(oxidanyl)phosphoryl]-1-oxidanyl-ethyl]-oxidanyl-phosphinate
| Molecular Weight | 249.99 g/mol |
|---|---|
| Molecular Formula | C2H6Na2O7P2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 249.93841509 g/mol |
| Monoisotopic Mass | 249.93841509 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 206 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Didronel |
| Active Ingredient | Etidronate disodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Procter And Gamble |
| 2 of 4 | |
|---|---|
| Drug Name | Etidronate disodium |
| PubMed Health | Etidronate (By mouth) |
| Drug Classes | Calcium Regulator |
| Drug Label | Etidronate disodium tablets, USP contain either 200 mg or 400 mg of etidronate disodium, the disodium salt of (1-hydroxyethylidene) diphosphonic acid, for oral administration. This compound, also known as EHDP, regulates bone metabolism. Etidronate d... |
| Active Ingredient | Etidronate disodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Didronel |
| Active Ingredient | Etidronate disodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Procter And Gamble |
| 4 of 4 | |
|---|---|
| Drug Name | Etidronate disodium |
| PubMed Health | Etidronate (By mouth) |
| Drug Classes | Calcium Regulator |
| Drug Label | Etidronate disodium tablets, USP contain either 200 mg or 400 mg of etidronate disodium, the disodium salt of (1-hydroxyethylidene) diphosphonic acid, for oral administration. This compound, also known as EHDP, regulates bone metabolism. Etidronate d... |
| Active Ingredient | Etidronate disodium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg |
| Market Status | Prescription |
| Company | Mylan |
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : DIDRONEL
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1982-01-01
Application Number : 17831
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : DIDRONEL
Dosage Form : TABLET;ORAL
Dosage Strength : 400MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1982-01-01
Application Number : 17831
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : DIDRONEL
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 50MG/ML
Approval Date : 1987-04-20
Application Number : 19545
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Brand Name : ETIDRONATE DISODIUM
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2003-01-24
Application Number : 75800
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Brand Name : ETIDRONATE DISODIUM
Dosage Form : TABLET;ORAL
Dosage Strength : 400MG
Approval Date : 2003-01-24
Application Number : 75800
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?