
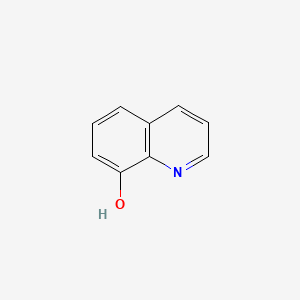

1. 8 Hydroxyquinoline
2. 8 Hydroxyquinoline Sulfate
3. 8 Oxyquinoline
4. 8 Quinolinol
5. 8-hydroxyquinoline Sulfate
6. 8-oxyquinoline
7. 8-quinolinol
8. Bioquin
9. Chinosol
10. Khinozol
11. Leioderm
12. Oxine
13. Oxyquinol
14. Oxyquinoline
15. Oxyquinoline Potassium Sulfate (2:1)
16. Oxyquinoline Sulfate
17. Quinosol
18. Sulfate, 8-hydroxyquinoline
19. Sulfate, Oxyquinoline
20. Superol
1. Quinolin-8-ol
2. 8-quinolinol
3. 148-24-3
4. Oxyquinoline
5. Oxine
6. Quinophenol
7. Oxychinolin
8. 8-quinol
9. 8-oxyquinoline
10. Phenopyridine
11. 8-hydroxychinolin
12. Bioquin
13. Oxybenzopyridine
14. Hydroxybenzopyridine
15. Oxin
16. 1-azanaphthalene-8-ol
17. Tumex
18. 8-chinolinol
19. 8-oq
20. 8-hydroxy-chinolin
21. Fennosan H 30
22. 8-oxychinolin
23. Fennosan
24. Oxyquinol
25. O-oxychinolin
26. Usaf Ek-794
27. Hydroxyquinoline
28. Oxyquinoline [usan]
29. 8-hydroxy-quinoline
30. Nci-c55298
31. Nsc 2039
32. Mfcd00006807
33. Oxoquinoline
34. Albisal
35. Nsc 615011
36. Nsc-2039
37. Nsc285166
38. 8-quinolinol, Homopolymer
39. Nsc-285166
40. Nsc-402623
41. Nsc-615011
42. Mls002702126
43. 5utx5635hp
44. Chembl310555
45. Chebi:48981
46. Nsc 82408
47. Quinoline-8-ol
48. Oxyquinoline (8-hydroxyquinoline)
49. Nsc 82404
50. Nsc-48037
51. Nsc-54230
52. Nsc-82404
53. Nsc-82405
54. Nsc-82409
55. Nsc-82410
56. Nsc-82412
57. Nsc615011
58. J2.960b
59. Oxyquinoline (usan)
60. Ncgc00090708-03
61. Ncgc00090708-05
62. Dsstox_cid_730
63. Dsstox_rid_75758
64. Wln: T66 Bnj Jq
65. Dsstox_gsid_20730
66. Oxine;8-hydroxyquinoline;quinophenol;8-quinolinone
67. Fennosan Hf-15
68. Caswell No. 719
69. 8-chinolinol [czech]
70. O-oxychinolin [german]
71. Quinoline, 8-hydroxy-
72. 84063-18-3
73. Cas-148-24-3
74. Manganese, Bis(8-quinolinolato)-
75. Smr000112313
76. 8-hydroxy-chinolin [german]
77. Ccris 340
78. 8-hydroxy Quinoline
79. Hsdb 4073
80. Einecs 205-711-1
81. Epa Pesticide Chemical Code 059803
82. Brn 0114512
83. Unii-5utx5635hp
84. Oxychinoline
85. Ai3-00483
86. 8-oxyquinolin
87. 8-quinolinone
88. 8-quinolol
89. 8-hydroxiquinoline
90. 8-hydroxychinoline
91. 8-hydroxylquinoline
92. Hqy
93. 8-quinolinol, P.a.
94. Spectrum_001053
95. 3vh9
96. 8-hydroxyquinoline Oxine
97. Spectrum2_000697
98. Spectrum3_000534
99. Spectrum4_000465
100. Spectrum5_001280
101. Oxyquinoline [ii]
102. 8-hydroxyquinoline, 99%
103. Ec 205-711-1
104. Ncimech_000694
105. Oxyquinoline [hsdb]
106. Oxyquinoline [inci]
107. Cid_1923
108. Ncistruc1_000152
109. Ncistruc2_000240
110. Oxyquinol Reference Spectrum
111. Nciopen2_000962
112. Nciopen2_001020
113. Nciopen2_001220
114. Nciopen2_004264
115. Oxyquinoline [vandf]
116. Schembl37189
117. Bspbio_002147
118. Kbiogr_000910
119. Kbioss_001533
120. 5-21-03-00252 (beilstein Handbook Reference)
121. Mls001055492
122. Bidd:er0371
123. Divk1c_000757
124. Spbio_000853
125. 8-hydroxyquinoline, Crystalline
126. Zinc8492
127. Hydroxyquinoline [vandf]
128. 8-hydroxyquinoline [mi]
129. Dtxsid5020730
130. Bdbm32203
131. Hms502f19
132. Kbio1_000757
133. Kbio2_001533
134. Kbio2_004101
135. Kbio2_006669
136. Kbio3_001647
137. Hydroxyquinoline [who-dd]
138. Nsc2039
139. 8-hydroxyquinoline [iarc]
140. Ninds_000757
141. 8-quinolinol (7ci,8ci,9ci)
142. Act08881
143. Hy-b1005
144. Str00721
145. Tox21_113083
146. Tox21_202986
147. Tox21_400006
148. 8-oxychinolin, 8-quinolinol, Oxine
149. Ccg-35870
150. Nsc 48037
151. Nsc 54230
152. Nsc 82405
153. Nsc 82409
154. Nsc 82410
155. Nsc 82412
156. Nsc402623
157. S4547
158. Stk943764
159. 8-hydroxyquinoline Acs Reagent Grade
160. Akos001061311
161. Ac-5109
162. Cs-4502
163. Db11145
164. Nsc 285166
165. Nsc 402623
166. Ps-4553
167. Sb40773
168. 8-hydroxyquinoline, Acs Reagent, 99%
169. Idi1_000757
170. Ncgc00090708-01
171. Ncgc00090708-02
172. Ncgc00090708-04
173. Ncgc00090708-06
174. Ncgc00090708-07
175. Ncgc00090708-08
176. Ncgc00090708-11
177. Ncgc00260531-01
178. Nci60_001712
179. Nci60_002335
180. 8-hydroxyquinoline Acs Grade 100g
181. Sbi-0051472.p003
182. Db-012222
183. Am20050821
184. Ft-0621550
185. H0305
186. 8-quinolinol, Jis Special Grade, >=99.0%
187. 8-quinolinol, Vetec(tm) Reagent Grade, 99%
188. En300-17403
189. C19434
190. D05321
191. P17615
192. Us9394254, 6
193. 8-quinolinol, Pestanal(r), Analytical Standard
194. Ab00052065_08
195. 8-quinolinol, >=99% (perchloric Acid Titration)
196. A808745
197. Ap-065/40180076
198. Q270162
199. Cu-01000012874-2
200. W-108106
201. Brd-k66808046-065-01-1
202. Z56926518
203. F0001-0526
204. 8-quinolinol, Puriss. P.a., Acs Reagent, For The Detection And Determination Of Al, Mg And Others, >=99.0% (nt)
205. 8-quinolinol, Puriss. P.a., Acs Reagent, Reag. Ph. Eur., >=99% (perchloric Acid Titration)
| Molecular Weight | 145.16 g/mol |
|---|---|
| Molecular Formula | C9H7NO |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 145.052763847 g/mol |
| Monoisotopic Mass | 145.052763847 g/mol |
| Topological Polar Surface Area | 33.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 138 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A BACTERIOSTATIC & FUNGISTATIC COMPOUND; USED PRINCIPALLY IN TREATMENT OF MINOR BURNS & OF HEMORRHOIDS.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1111
OXYQUINOLINE SULFATE ... IS ... USED ... IN TREATMENT OF ATHLETE'S FOOT, VAGINITIS, & AS A GARGLE, EYEWASH, NASAL DOUCHE, & IN HEMORRHOIDAL PREPARATIONS ... /OXYQUINOLINE SULFATE/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1111
/OVER THE COUNTER/ HYDROXYQUINOLINE IS 1 OF 4 ANTIFUNGAL AGENTS RECOMMENDED FOR ACTIVE TREATMENT OF FUNGUS ASSOCIATED WITH DIAPER RASH & PRICKLY HEAT IN BABIES. /HYDROXYQUINOLINE/
SADIK F; J AM PHARM ASSOC NS10 (JAN): 19-24 (1970)
8-HYDROXYQUNIOLINE SULFATE INHIBITED FORMATION OF ARTIFICIAL CALCULUS IN VITRO & RAT CALCULUS IN VIVO. IN RATS, IT PREVENTED CALCULUS FORMATION WHEN APPLIED BY SWABBING OR BY INTRAORAL INSTILLATION. IN DOGS, FORMATION OF DENTAL PLAQUE WAS INHIBITED 33 TO 98% IN COMPARISON TO PLACEBO. ALSO, 25 TO 58% OF ESTABLISHED PLAQUE ACCUMULATIONS WERE REMOVED, WHEREAS PLACEBO REMOVED 2 TO 22%.
PMID:815301 DEPALMA PD ET AL; J DENT RES 55 (2): 292-8 (1976)
For more Therapeutic Uses (Complete) data for 8-HYDROXYQUINOLINE (7 total), please visit the HSDB record page.
Oxyquinoline is used as a biocidal component of several over the counter products. These products are marketed for the purposes of inhibiting abnormal biological growth in the vagina and restoring natural pH.
Oxyquinoline acts as a biocide to eliminate bacteria and fungi.
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AB - Antiinfectives and antiseptics for local oral treatment
A01AB07 - Oxyquinoline
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AH - Quinoline derivatives
D08AH03 - Oxyquinoline
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AC - Quinoline derivatives
G01AC30 - Oxyquinoline
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA14 - Oxyquinoline
Route of Elimination
Oxyquinoline is excreted in both the primarily in the urine with some in the bile.
IN RATS /MALE, DONRYU STRAIN, IV INJECTION/ 8-HYDROXYQUINOLINE WAS METABOLIZED TO GLUCURONIDE & SULFATE CONJUGATES. MORE 8-HYDROXYQUINOLINE GLUCURONIDE WAS EXCRETED IN URINE THAN 8-HYDROXYQUINOLINE SULFATE CONJUGATE. ONLY THE GLUCURONIDE CONJUGATE WAS EXCRETED IN BILE.
PMID:409509 KIWADA H ET AL; CHEM PHARM BULL 25 (7): 1566-73 (1977)
8-HYDROXYQUINOLINE WAS METABOLIZED TO GLUCURONIDE & SULFATE CONJUGATES AFTER IV ADMIN IN RATS /MALE, DONRYU STRAIN/. THE GLUCURONIDES WERE EXCRETED IN BILE & URINE, BUT THE SULFATES WERE EXCRETED EXCLUSIVELY IN THE URINE. UNMETABOLIZED FORMS WERE ONLY SLIGHTLY EXCRETED.
PMID:96945 SAWADA Y ET AL; CHEM PHARM BULL 26 (5): 1357-63 (1978)
In the urine, 60% of the dose is excreted as glucuronide conjugates and 23% of the dose as sulfate conjugates. In the bile, 9% of the total dose is found as glucuronide conjugates.
IN RATS /MALE, DONRYU STRAIN, IV INJECTION/ 8-HYDROXYQUINOLINE WAS METABOLIZED TO GLUCURONIDE & SULFATE CONJUGATES.
PMID:409509 KIWADA H ET AL; CHEM PHARM BULL 25 (7): 1566-73 (1977)
8-HYDROXYQUINOLINE WAS METABOLIZED TO GLUCURONIDE & SULFATE CONJUGATES AFTER IV ADMIN IN RATS /MALE, DONRYU STRAIN/. UNMETABOLIZED FORMS WERE ONLY SLIGHTLY EXCRETED.
PMID:96945 SAWADA Y ET AL; CHEM PHARM BULL 26 (5): 1357-63 (1978)
The mechanism by which oxyquinoline exerts its biocidal effect is unknown.
BUILDING BLOCK

Evonik's CDMO solutions for APIs and HPAPIs: It specializes where the client needs it most!
MARKET PLACE

