
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
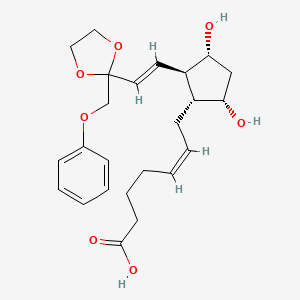

1. Etiproston Tromethamine
1. 59619-81-7
2. Etiprostone
3. Etiproston [inn]
4. Prostavet
5. Tcu22w0apy
6. (z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(e)-2-[2-(phenoxymethyl)-1,3-dioxolan-2-yl]ethenyl]cyclopentyl]hept-5-enoic Acid
7. Etiproston (inn)
8. (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((e)-2-(2-(phenoxymethyl)-1,3-dioxolan-2-yl)vinyl)cyclopentyl)-5-heptenoic Acid
9. Etiprostonum
10. Etiprostone [inn-french]
11. Unii-tcu22w0apy
12. Etiprostonum [inn-latin]
13. Brn 1274276
14. Etiproston [mi]
15. Prostavet [veterinary] (tn)
16. Chembl2104285
17. Schembl11602088
18. Dtxsid501024232
19. Zinc30691625
20. 15-deoxy-15,15-ethylenedioxy-16-phenoxy-17,18,19,20-tetranor-pgf2-alpha
21. 15-deoxy-15,15-ethylenedioxy-16-phenoxy-17,18,19,20-tetranor-prostaglandin F2-alpha
22. D07932
23. 619e817
24. Q27289904
25. 15-deoxy-15, 15-ethylenedioxy-16-phenoxy-omega-tetranor Pgf-2alpha
26. 5-heptenoic Acid, 7-(3,5-dihydroxy-2-(2-(2-(phenoxymethyl)-1,3-dioxolan-2-yl)ethenyl)cyclopentyl)-, (1r-(1-alpha(z),2-beta(e),3-alpha,5-alpha))-
| Molecular Weight | 432.5 g/mol |
|---|---|
| Molecular Formula | C24H32O7 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 432.21480336 g/mol |
| Monoisotopic Mass | 432.21480336 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 605 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?