
Synopsis
Synopsis
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
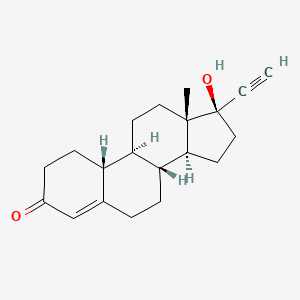

1. 19-norpregn-4-en-20-yn-3-one, 17-hydroxy-, (17alpha)-
2. Conceplan
3. Ethinylnortestosterone
4. Micronor
5. Monogest
6. Nor Qd
7. Nor-qd
8. Norcolut
9. Norcolute
10. Norethindrone, (1 Beta)-isomer
11. Norethisterone
12. Norlutin
13. Norpregneninolone
14. Norqd
1. Norethisterone
2. 68-22-4
3. Micronor
4. Norethisteron
5. 19-norethisterone
6. Norlutin
7. Anovule
8. Gestest
9. Norcolut
10. Noriday
11. Utovlan
12. Camila
13. Primolut-n
14. Norethynodrone
15. Conludag
16. Micronovum
17. Norluten
18. Triella
19. Mini-pill
20. Nor-qd
21. Mini-pe
22. Noresthisterone
23. Norethisteronum
24. Conludaf
25. Micronett
26. Noralutin
27. Norethyndron
28. Norgestin
29. Norluton
30. Proluteasi
31. Norfor
32. Utovlar
33. Norpregneninlone
34. 19-nor-ethindrone
35. Ethinylnortestosterone
36. Noretisterona
37. 17alpha-ethynyl-19-nortestosterone
38. Ethynylnortestosterone
39. 19-nor-17alpha-ethynyltestosterone
40. Menzol
41. 17alpha-ethinyl-19-nortestosterone
42. Anhydrohydroxynorprogesterone
43. Minovlar
44. Nor-q.d.
45. 19-norethinyltestosterone
46. Norpregneninolone
47. Ethinyl-19-nortestosterone
48. Normapause
49. 17alpha-ethynyl-4-estren-17-ol-3-one
50. 17-alpha-ethynyl-19-nortestosterone
51. 19-nor-17-alpha-ethynyltestosterone
52. 19-norethindrone
53. 19-nor-17-ethinyltestosterone
54. 17alpha-ethinylestra-4-en-17beta-ol-3-one
55. 17beta-hydroxy-19-norpregn-4-en-20-yn-3-one
56. Nsc-9564
57. 17-alpha-ethynyl-4-estren-17-ol-3-one
58. 19-nor-17alpha-ethynyl-17beta-hydroxy-4-androsten-3-one
59. 17alpha-ethynyl-17-hydroxy-4-estren-3-one
60. Activella
61. 19-nor-17alpha-ethynylandrosten-17beta-ol-3-one
62. 4-estren-17alpha-ethynyl-17beta-ol-3-one
63. 17alpha-ethynyl-19-norandrost-4-en-17beta-ol-3-one
64. 17-hydroxy-19-nor-17alpha-pregn-4-en-20-yn-3-one
65. 17-alpha-ethynyl-17-hydroxy-4-estren-3-one
66. 17alpha-ethynyl-17beta-hydroxy-19-norandrost-4-en-3-one
67. 17-beta-hydroxy-19-norpregn-4-en-20-yn-3-one
68. Norethindrone (usp)
69. Norethindrone [usp]
70. Primolut N
71. Norethisterone [inn]
72. 19-nor-17-alpha-ethynylandrosten-17-beta-ol-3-one
73. 17-ethynyl-17beta-hydroxyestr-4-en-3-one
74. 17-alpha-ethynyl-19-norandrost-4-en-17-beta-ol-3-one
75. 17-hydroxy-19-nor-17-alpha-pregn-4-en-20-yn-3-one
76. Anovulatorio
77. Ciclovulan
78. Microneth
79. Norethadrone
80. Norethynodron
81. 19-nor-17-alpha-ethynyl-17-beta-hydroxy-4-androsten-3-one
82. Estrinor
83. Gencept
84. Norethin
85. Synphase
86. Genora
87. Milli
88. Nelova
89. Nodiol
90. Noraethisteronum
91. Norpregneninotone
92. Chembl1162
93. Combipatch
94. Norcept-e
95. 17alpha-ethynyl-19-nor-4-androsten-17beta-ol-3-one
96. Norethindrone (norethisterone)
97. Synphasic 28
98. (17-alpha)-17-hydroxy-19-norpregn-4-en-20-yn-3-one
99. Brevinor 21
100. Brevinor 28
101. Chebi:7627
102. Trinovum 21
103. Noriday 28
104. Errin
105. Jenest-28
106. Ethynylmortestosterone
107. Brevinor-1 21
108. Brevinor-1 28
109. T18f433x4s
110. Tri-norinyl
111. Ovysmen 1 35
112. Noretisterone [dcit]
113. Ortho-novum 1 35
114. Ortho-novum 1 50
115. Sc 4640
116. Ortho-novum 7 7 7
117. Ovysmen 0.5 35
118. Ortho 1 35
119. (8r,9s,10r,13s,14s,17r)-17-ethynyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one
120. Sc-4640
121. Ortho 7 7 7
122. Noretisterone
123. 19-nortestosterone, 17-ethynyl-
124. Norethisteronum [inn-latin]
125. Noretisterona [inn-spanish]
126. Dsstox_cid_3380
127. Dsstox_rid_77005
128. 17alpha-hydroxy-19-norpregn-4-en-20-yn-3-one
129. Dsstox_gsid_23380
130. Nora-be
131. Norethisterone [progestins]
132. (14beta,17alpha)-17-ethynyl-17-hydroxyestr-4-en-3-one
133. Norethindirone
134. 17-ethinyl-19-nortestosterone
135. (17alpha)-17-ethynyl-17-hydroxyestra-4,8(14),9-trien-3-one
136. 19-nor-17alpa-ethynyltestosterone
137. Micronor (tn)
138. (8r,9s,10r,13s,14s,17r)-17-ethynyl-17-hydroxy-13-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3h-cyclopenta[a]phenanthren-3-one
139. Smr000499579
140. Ccris 484
141. Norethisterone (norethindrone)
142. Primolut-n (tn)
143. Camila (tn)
144. N.e.e.
145. 19-nor-17-ethinyl Testosterone
146. Hsdb 3370
147. 19-nor-ethinyl--4,5-testosterone
148. Einecs 200-681-6
149. Brn 1915671
150. 19-norpregn-4-en-20-yn-3-one, 17-hydroxy-, (17.alpha.)-
151. Heather Whole
152. Unii-t18f433x4s
153. Ai3-26422
154. 17-alpha-ethinylestra-4-en-17-beta-ol-3-one
155. Cas-68-22-4
156. Estr-4-en-3-one, 17alpha-ethynyl-17-hydroxy-
157. Ncgc00094738-01
158. Ent
159. Net
160. Prestwick_646
161. Calluna Vulgaris Whole
162. 17-alpha-19-norpregn-4-en-20-yn-3-one, 17-hydroxy-
163. 17alpha-ethinyl-17beta-hydroxy-delta(sup:4)-estren-3-one
164. 19-nor-17alpha-pregn-4-en-20-yn-3-one, 17-hydroxy-
165. 17-alpha-ethinyl-17-beta-hydroxy-delta(sup:4)-estren-3-one
166. 17-alpha-ethynyl-17-beta-hydroxy-19-norandrost-4-en-3-one
167. Norethisterone (jp17)
168. Prestwick0_000253
169. Prestwick1_000253
170. Prestwick2_000253
171. Prestwick3_000253
172. Norethindrone [mi]
173. 17-hydroxy-19-nor-17.alpha.-pregn-4-en-20-yn-3-one
174. Ec 200-681-6
175. Norethindrone [hsdb]
176. Norethisterone [jan]
177. Schembl23390
178. Bspbio_000066
179. Levonorgestrel Ep Impurity U
180. Norethindrone [vandf]
181. Mls001076679
182. Mls001163874
183. Norethisterone / Norethindrone
184. Spbio_002285
185. Norethindrone [usp-rs]
186. Norethisterone [mart.]
187. Bpbio1_000074
188. Gtpl2880
189. 17-ethynyl-19-nortestosterone
190. Norethisterone [who-dd]
191. Norethisterone [who-ip]
192. (17alpha)-17-hydroxy-19-norpregn-4-en-20-yn-3-one
193. Dtxsid9023380
194. 17-ethinyl-19-nor-testosterone
195. 19-nor-17-alpha-pregn-4-en-20-yn-3-one, 17-hydroxy-
196. Hms1568d08
197. Hms2090d21
198. Hms2095d08
199. Hms2231e18
200. Hms3259c16
201. Hms3712d08
202. Norethindrone [orange Book]
203. Bcp28306
204. Hy-b0554
205. 17alpha-pregn-4-en-20-yn-3-one
206. Norethindrone [usp Impurity]
207. Norethisterone [ep Impurity]
208. Tox21_111322
209. Tox21_302427
210. 19-norethindrone, >=98%, Powder
211. Bdbm50148732
212. Lmst02030097
213. Norethindrone [usp Monograph]
214. S4040
215. Zinc85205451
216. Norinyl Component Norethindrone
217. Vyfemla Component Norethindrone
218. Akos005267172
219. Aranelle Component Norethindrone
220. Brevicon Component Norethindrone
221. Norethisteronum [who-ip Latin]
222. Taytulla Component Norethindrone
223. Tox21_111322_1
224. Ccg-220253
225. Db00717
226. Nc00576
227. Femcon Fe Component Norethindrone
228. Ncgc00179669-01
229. Ncgc00179669-02
230. Ncgc00179669-04
231. Ncgc00255187-01
232. Ac-11100
233. Ac-33117
234. As-56451
235. Cpd000499579
236. Norethindrone Component Of Aranelle
237. Norquest Fe Component Norethindrone
238. 17alpha-ethynyl-17-hydroxyest-4-en-3-one
239. 17alpha-ethynyl-3-oxo-4-estren-17beta-ol
240. Norethindrone Component Of Femcon Fe
241. N0449
242. Norethindrone Component Of Norquest Fe
243. 17alpha-ethynyl-17-hydroxy-estr-4-en-3-one
244. Lo Minastrin Fe Component Norethindrone
245. Norethisterone 100 Microg/ml In Acetonitrile
246. C05028
247. C76161
248. D00182
249. Levonorgestrel Impurity U [ep Impurity]
250. Minastrin 24 Fe Component Norethindrone
251. 17alpha-ethynyl-17beta-hydroxyestr-4-en-3-one
252. 17beta-hydroxy-17alpha-ethynylestr-4-en-3-one
253. 067n596
254. 17-alpha-ethynyl-17-beta-hydroxy-4-estren-3-one
255. A836053
256. Q421352
257. Sr-01000765382
258. 17alpha-ethinyl-17alpha-ethinyl-19-nortestosterone
259. Sr-01000765382-3
260. W-104686
261. 13-methyl-17alpha-ethynyl-17-hydroxygon-4-en-3-one
262. 17-hydroxy-17-alpha-19-norpregn-4-en-20-yn-3-one
263. 17-hydroxy-19-nor-17alpha-pregn-4-en-20yn-3-one
264. 19-norethindrone, Vetranal(tm), Analytical Standard
265. Brd-k92073408-001-03-3
266. Brd-k92073408-001-16-5
267. Norethisterone Acetate Impurity A [ep Impurity]
268. 17-hydroxy-(17alpha)-19-norpregn-4-en-20-yn-3-one
269. 17-ethynyl-17-hydroxyestr-4-en-3-one (acd/name 4.0)
270. Norethisterone, British Pharmacopoeia (bp) Reference Standard
271. Norethisterone, European Pharmacopoeia (ep) Reference Standard
272. Norethindrone, United States Pharmacopeia (usp) Reference Standard
273. Norethisterone For System Suitability, European Pharmacopoeia (ep) Reference Standard
274. Norethindrone (norethisterone), Pharmaceutical Secondary Standard; Certified Reference Material
275. Norethisterone; 19-norethisterone; 17?-ethynyl-19-nortestosterone; 17-hydroxy-19-nor-17?-pregn-4-en-20-yn-3-one
| Molecular Weight | 298.4 g/mol |
|---|---|
| Molecular Formula | C20H26O2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 298.193280068 g/mol |
| Monoisotopic Mass | 298.193280068 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 594 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Activella |
| PubMed Health | Estradiol/Norethindrone |
| Drug Classes | Contraceptive, Estrogen/Progestin Combination, Monophasic Contraceptive Combination |
| Active Ingredient | norethindrone acetate; Estradiol |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.5mg; 1mg; 1mg/0.5mg; 0.1mg |
| Market Status | Prescription |
| Company | Amneal Pharms; Novo Nordisk |
| 2 of 12 | |
|---|---|
| Drug Name | Camila |
| PubMed Health | Norethindrone (By mouth) |
| Drug Classes | Contraceptive, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Each light pink Camila tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone USP daily, and has the following inactive ingredients: corn starch, FD&C red no. 40 aluminum lake, lactose monohydrate, magnesium stearate, povi... |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Barr |
| 3 of 12 | |
|---|---|
| Drug Name | Errin |
| PubMed Health | Norethindrone (By mouth) |
| Drug Classes | Contraceptive, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Barr |
| 4 of 12 | |
|---|---|
| Drug Name | Micronor |
| PubMed Health | Norethindrone (By mouth) |
| Drug Classes | Contraceptive, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Each tablet contains 0.35 mg norethindrone. Inactive ingredients include corn starch, D&C Green No. 5, D&C Yellow No. 10, lactose, magnesium stearate, and povidone.... |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 5 of 12 | |
|---|---|
| Drug Name | Norethindrone |
| Drug Label | Each light pink Camila tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone USP daily, and has the following inactive ingredients: corn starch, FD&C red no. 40 aluminum lake, lactose monohydrate, magnesium stearate, povi... |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Novast Labs; Lupin; Famy Care; Glenmark Generics; Haupt Pharma |
| 6 of 12 | |
|---|---|
| Drug Name | Nor-qd |
| Drug Label | Each yellow Nor-QD tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone daily, and the inactive ingredients include D&C Yellow No. 10, FD&C Yellow No. 6, lactose, magnesium stearate, povidone, and starch.The chemical na |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Watson Labs (utah) |
| 7 of 12 | |
|---|---|
| Drug Name | Activella |
| PubMed Health | Estradiol/Norethindrone |
| Drug Classes | Contraceptive, Estrogen/Progestin Combination, Monophasic Contraceptive Combination |
| Active Ingredient | norethindrone acetate; Estradiol |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.5mg; 1mg; 1mg/0.5mg; 0.1mg |
| Market Status | Prescription |
| Company | Amneal Pharms; Novo Nordisk |
| 8 of 12 | |
|---|---|
| Drug Name | Camila |
| PubMed Health | Norethindrone (By mouth) |
| Drug Classes | Contraceptive, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Each light pink Camila tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone USP daily, and has the following inactive ingredients: corn starch, FD&C red no. 40 aluminum lake, lactose monohydrate, magnesium stearate, povi... |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Barr |
| 9 of 12 | |
|---|---|
| Drug Name | Errin |
| PubMed Health | Norethindrone (By mouth) |
| Drug Classes | Contraceptive, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Barr |
| 10 of 12 | |
|---|---|
| Drug Name | Micronor |
| PubMed Health | Norethindrone (By mouth) |
| Drug Classes | Contraceptive, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Each tablet contains 0.35 mg norethindrone. Inactive ingredients include corn starch, D&C Green No. 5, D&C Yellow No. 10, lactose, magnesium stearate, and povidone.... |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 11 of 12 | |
|---|---|
| Drug Name | Norethindrone |
| Drug Label | Each light pink Camila tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone USP daily, and has the following inactive ingredients: corn starch, FD&C red no. 40 aluminum lake, lactose monohydrate, magnesium stearate, povi... |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Novast Labs; Lupin; Famy Care; Glenmark Generics; Haupt Pharma |
| 12 of 12 | |
|---|---|
| Drug Name | Nor-qd |
| Drug Label | Each yellow Nor-QD tablet provides a continuous oral contraceptive regimen of 0.35 mg norethindrone daily, and the inactive ingredients include D&C Yellow No. 10, FD&C Yellow No. 6, lactose, magnesium stearate, povidone, and starch.The chemical na |
| Active Ingredient | Norethindrone |
| Dosage Form | Tablet |
| Route | Oral-28 |
| Strength | 0.35mg |
| Market Status | Prescription |
| Company | Watson Labs (utah) |
Contraceptives, Oral, Synthetic; Progestational Hormones, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
AYGESTIN is indicated for the treatment of secondary amenorrhea, endometriosis, and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer. AYGESTIN is not intended, recommended or approved to be used with concomitant estrogen therapy in postmenopausal women for endometrial protection. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for AYGESTIN (norethindrone acetate) tablet (July 2007). Available from, as of February 14, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=21992
Oral contraceptives are indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for BALZIVA (norethindrone and ethyl estradiol) kit (September 2009). Available from, as of February 28, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11472
Progestin-only oral contraceptives are indicated for the prevention of pregnancy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for CAMILA (norethindrone) tablet (January 2011). Available from, as of February 28, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=37602
For more Therapeutic Uses (Complete) data for NORETHINDRONE (6 total), please visit the HSDB record page.
VET: MARKED RAT, MONKEY, & DOG FETAL MASCULINIZATION HAS BEEN OBSERVED AS IN HUMAN. EXCESSIVE DOSAGE DURING PREGNANCY MAY ALSO CAUSE FETAL DEATH, TERATOGENICITY, & DELAYED PARTURITION.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 394
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
US Natl Inst Health; DailyMed. Current Medication Information for BALZIVA (norethindrone and ethyl estradiol) kit (September 2009). Available from, as of February 28, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11472
Norethindrone, like other progestins, may cause breakthrough bleeding, spotting, changes in menstrual flow, amenorrhea, changes in cervical erosion and secretions, edema, weight gain or loss, cholestatic jaundice, allergic rash with or without pruritus, melasma or chloasma, and mental depression.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3278
When breakthrough bleeding or irregular vaginal bleeding occurs during norethindrone therapy, nonfunctional causes should be considered. Adequate diagnostic procedures should be performed in patients with undiagnosed vaginal bleeding.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3278
For more Drug Warnings (Complete) data for NORETHINDRONE (44 total), please visit the HSDB record page.
3(?). 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT FOR 70 KG PERSON (150 LB). /NORETHINDRONE WITH MESTRANOL/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-174
Norethisterone is indicated as an oral contraceptive when given as monotherapy or in combination with an estrogen component, such as [ethinylestradiol] or [estradiol]. In combination with an estrogen component, oral norethisterone is also indicated as a hormone replacement therapy in the treatment of postmenopausal osteoporosis and moderate-to-severe vasomotor symptoms arising from menopause. When applied via transdermal patch, the combination of norethisterone and estradiol is indicated for the treatment of hypoestrogenism, vulvovaginal atrophy, and moderate-severe vasomotor symptoms. Norethisterone, taken in combination with intramuscular [leuprolide], is also indicated for the symptomatic treatment of endometriosis-related pain.
Norethisterone is a synthetic oral progestin used for contraception or to treat other hormone-related conditions such as menopausal symptoms and endometriosis. As a synthetic progestin, norethisterone acts similarly to endogenous progesterone but with a much higher potency - it acts at the pelvic level to alter cervical and endometrial function, as well as via the inhibition of pituitary hormones that play a role in follicular maturation and ovulation. A small increase in the risk of developing breast cancer has been observed in patients using combined oral contraceptives, with some evidence also implicating progestin-only pills - patients starting hormonal contraception should be advised of this risk and should employ routine breast self-examinations to check for evidence of any developing masses.
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
Contraceptives, Oral, Hormonal
Oral contraceptives which owe their effectiveness to hormonal preparations. (See all compounds classified as Contraceptives, Oral, Hormonal.)
G03FA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03A - Hormonal contraceptives for systemic use
G03AC - Progestogens
G03AC01 - Norethisterone
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03D - Progestogens
G03DC - Estren derivatives
G03DC02 - Norethisterone
Absorption
The Cmax of norethisterone following oral administration of a single dose ranges from 5.39 to 7.36 ng/mL with a Tmax of 1-2 hours. AUC0-24 values following single oral doses range from approximately 30 to 37 ng*hr/mL. The oral bioavailability of norethisterone is approximately 64%. When applied transdermally, norethisterone is well-absorbed through the skin, reaches steady-state concentrations within 24 hours, and has a Cmax ranging from 617 to 1060 pg/mL at steady state. Norethisterone is often formulated as norethisterone acetate, which is completely and rapidly deacetylated to norethisterone following oral administration - the disposition of norethisterone acetate is indistinguishable from that of orally administered norethisterone.
Route of Elimination
Following administration of radio-labeled norethisterone, slightly more than 50% of the administered dose was eliminated in the urine and 20-40% was eliminated in the feces.
Volume of Distribution
The volume of distribution of norethisterone is approximately 4 L/kg. Sulfated metabolites of norethisterone, as well as small quantities of parent drug, have been shown to distribute into breast milk.
Clearance
The plasma clearance of norethisterone has been estimated as 0.4 L/hr/kg, and the intrinsic clearance is approximately 73-81 L/h.
Norethindrone is 36% bound to sex hormone-binding globulin (SHBG) and 61% bound to albumin. Volume of distribution of norethindrone is about 4 L/kg.
US Natl Inst Health; DailyMed. Current Medication Information for AYGESTIN (norethindrone acetate) tablet (July 2007). Available from, as of February 14, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=21992
Plasma clearance value for norethindrone is approximately 0.4 L/hr/kg.
US Natl Inst Health; DailyMed. Current Medication Information for AYGESTIN (norethindrone acetate) tablet (July 2007). Available from, as of February 14, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=21992
In 25 BALB/c mice implanted subcutaneously with pellets containing 40% norethisterone and 60% cholesterol for 76-77 wk, absorption of norethisterone was estimated to be between 3.6 and 15.9 ug/day (mean, 7.7 ug/day).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 452 (1979)
Rabbits excrete norethisterone metabolites predominantly in the urine ... while rats excrete them to 80% in bile ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 453 (1979)
For more Absorption, Distribution and Excretion (Complete) data for NORETHINDRONE (8 total), please visit the HSDB record page.
Norethisterone is extensively metabolized, primarily in the liver, to a number of metabolites via partial and total reduction of its A-ring. The enzymes predominantly involved are 3- and 3-hydroxysteroid dehydrogenase (HSD) as well as 5- and 5-reductase. The 5-reduced metabolites, including 5-dihydronorethisterone and its derivatives, appear to carry biological activity while the 5-reduced metabolites appear inactive. Norethisterone and its metabolites are also extensively conjugated - most of the plasmatic metabolites are sulfate conjugates, while most of the urinary metabolites are glucuronide conjugates. The major metabolites in plasma are a disulfate conjugate of 3,5-tetrahydronorethisterone and a monosulfate conjugate of 3,5-tetrahydronorethisterone, while the major metabolite(s) in the urine are comprised of glucuronide and/or sulfate conjugates of 3,5-tetrahydronorethisterone. Norethisterone has also been observed to undergo some degree of metabolism via the cytochrome P450 enzyme system, predominantly by CYP3A4 and, to a much lesser extent, by CYP2C19, CYP1A2, and CYP2A6. The metabolites generated by these reactions have not been fully characterized.
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for AYGESTIN (norethindrone acetate) tablet (July 2007). Available from, as of February 14, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=21992
Norethisterone undergoes extensive ring A reduction to form dihydro- and tetrahydronorethisterone metabolites that undergo conjugation; it can also be aromatized. Low serum levels of ethinylestradiol have been measured in postmenopausal women following oral administration of relatively large doses of norethisterone acetate or norethisterone. On the basis of the area-under-the-curve (AUC) values that were determined for ethinylestradiol and norethisterone, it was shown that the mean conversion ratio of norethisterone to ethinylestradiol was 0.7 and 1.0% at doses of 5 and 10 mg, respectively. The authors calculated that this corresponds to an oral dose equivalent of about 6 ug ethinylestradiol/ mg of norethisterone acetate. Similarly, it was shown that a dose of 5 mg norethisterone administered orally was equivalent to about 4 ug ethinylestradiol/mg norethisterone.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 145 (2007)
After incubation of norethisterone with dog liver microsomes the 4beta,5beta-epoxide of norethisterone and a 6-oxygenated norethisterone derivative were obtained as minor metabolites ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 453 (1979)
Rabbit liver homogenates ... catalyze the deethinylation of norethisterone, giving rise to the metabolite oestr-4-ene-3,17-dione.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V21 453 (1979)
For more Metabolism/Metabolites (Complete) data for NORETHINDRONE (7 total), please visit the HSDB record page.
Norethindrone has known human metabolites that include Norethindrone-O-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of norethisterone has been variably estimated as 8-10 hours.
The mean terminal elimination half-life of norethindrone following a single dose administration of AYGESTIN is approximately 9 hours.
US Natl Inst Health; DailyMed. Current Medication Information for AYGESTIN (norethindrone acetate) tablet (July 2007). Available from, as of February 14, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=21992
The plasma half-life, MCR and plasma metabolite levels at various time intervals have been studied in six women after an intravenous injection of 3H-norethindrone acetate. The disappearance curve due to norethindrone acetate showed an initial rapid disappearance of 3H with an average half-life of 7.5 minutes and a subsequent slow disapperance with a half-life of 51.5 hours. Norethindrone acetate was cleared from the plasma with an average MCR of 495 L/day. Norethindrone acetate is rapidly metabolised after an intravenous injection. Norethindrone, the main metabolite, disappears from the plasma with an average half-life of 34.8 hours. Norethindrone maintains a high level compared with norethindrone acetate at all time intervals up to 24 hours and an equilibrium is reached between the two at 24 to 48 hours.
PMID:484634 Singh H et al; Am J Obstet Gynecol 135 (3): 409-14 (1979)
The half-life (of the beta phase of a two-component model) of elimination ranged from 4.8 to 12.8 hr (mean, 7.6 hr) with no significant differences between oral and intravenous administration.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 145 (2007)
On a molecular level, progestins like norethisterone exert their effects on target cells via binding to progesterone receptors that result in downstream changes to target genes. Target cells are found in the reproductive tract, breast, pituitary, hypothalamus, skeletal tissue, and central nervous system. Contraceptive efficacy is derived mainly from changes to the cervical mucus, wherein norethisterone increases the cell content and viscosity of the mucous to impede sperm transport and migration. Norethisterone also induces a variety of changes to the endometrium - including atrophy, irregular secretion, and suppressed proliferation - that make it inhospitable for implantation. Working via a negative feedback loop, norethisterone also acts on both the hypothalamus and anterior pituitary to suppress the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. Suppression of these hormones prevents follicular development, ovulation, and corpus luteum development. When used as a component of hormone replacement therapy in menopausal women, norethisterones value is mainly in suppressing the growth of the endometrium. As estrogen stimulates endometrial growth, the unopposed use of estrogen in postmenopausal women with an intact uterus can lead to endometrial hyperplasia which can increase the risk of endometrial cancer. The addition of a progestin to a hormone replacement therapy in this population protects against this endometrial hyperplasia and, therefore, lowers the risk associated with the use of hormone replacement therapies. Norethisterone, along with other progestins and endogenous progesterone, has a low affinity for other steroid receptors, such as the androgen receptor and glucocorticoid receptor. While affinity and agonistic activity at these receptors is minimal, it is thought that androgen receptor agonism is responsible for some of the adverse effects observed with progestin use (e.g. acne, serum lipid changes).
Norethindrone shares the actions of progestins. Although the exact mechanism of action of progestin-only oral contraceptives is not known, norethindrone, when administered in usual contraceptive doses, appears to act principally by altering cervical mucus so that sperm migration into the uterus is inhibited. Progestational changes in the endometrium also occur which may inhibit implantation of the fertilized ovum in the uterus. In addition, continuous administration of low doses of norethindrone alters the rate of ovum transport by changing motility and secretion in fallopian tubes. Norethindrone prevents pregnancy even in the presence of ovulation. Norethindrone suppresses ovulation and causes ovarian and endometrial atrophy at high doses; the drug does not consistently suppress ovulation when administered in a continuous low-dose regimen. In low doses, norethindrone causes variable suppression of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Norethindrone has mild androgenic activity. At low doses, norethindrone also has some estrogenic activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3119
Norethindrone shares the pharmacologic actions of the progestins. In women with adequate endogenous estrogen, norethindrone transforms a proliferative endometrium into a secretory one. Norethindrone has been shown to have some estrogenic, androgenic, and anabolic activity. The drug inhibits the secretion of pituitary gonadotropins at usual dosages and thus prevents follicular maturation and ovulation.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3279
Progestins enter target cells by passive diffusion and bind to cytosolic (soluble) receptors that are loosely bound in the nucleus. The steroid receptor complex initiates transcription, resulting in an increase in protein synthesis. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
Progestins are capable of affecting serum concentrations of other hormones, particularly estrogen. Estrogenic effects are modified by the progestins, either by reducing the availability or stability of the hormone receptor complex or by turning off specific hormone-responsive genes by direct interaction with the progestin receptor in the nucleus. In addition, estrogen priming is necessary to increase progestin effects by upregulating the number of progestin receptors and/or increasing progesterone production, causing a negative feedback mechanism that inhibits estrogen receptors. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
For more Mechanism of Action (Complete) data for NORETHINDRONE (9 total), please visit the HSDB record page.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : ACTILLETS™ are microcrystalline cellulose spheres used in advanced drug formulations as starter cores for drug layering and coating.
Pharmacopoeia Ref : NA
Technical Specs : Bulk density: 0.80
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Chewable & Orodispersible Aids
Excipient Details : CS90 is a directly compressible calcium carbonate with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-175, Tapped Density: 0.85
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Chewable & Orodispersible Aids
Excipient Details : MS90 is a directly compressible magnesium hydroxide with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-170, Taped Density: 0.80
Ingredient(s) : Starch
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Emulsifying Agents
Excipient Details : HDK N20 Pharma is used as a pharmaceutical emulsifying agent in tablets, capsules, syrups, and solutions.
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Brand Name : Titanium dioxide PRETIOX ...
Application : Coating Systems & Additives
Excipient Details : Titanium dioxide Pretiox AV01FG is used as a coloring and coating agent in oral solid dosage forms such as capsules, tablets, granules, and pellets.
Pharmacopoeia Ref : Fami-QS, Kosher, Halal, OHSAS ...
Technical Specs : Ti 59.95% and O 40.05%
Ingredient(s) : Titanium Dioxide
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
A 17.alpha.-Ethynyl-19-nortestosterone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 17.alpha.-Ethynyl-19-nortestosterone, including repackagers and relabelers. The FDA regulates 17.alpha.-Ethynyl-19-nortestosterone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 17.alpha.-Ethynyl-19-nortestosterone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 17.alpha.-Ethynyl-19-nortestosterone supplier is an individual or a company that provides 17.alpha.-Ethynyl-19-nortestosterone active pharmaceutical ingredient (API) or 17.alpha.-Ethynyl-19-nortestosterone finished formulations upon request. The 17.alpha.-Ethynyl-19-nortestosterone suppliers may include 17.alpha.-Ethynyl-19-nortestosterone API manufacturers, exporters, distributors and traders.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 17.alpha.-Ethynyl-19-nortestosterone DMF (Drug Master File) is a document detailing the whole manufacturing process of 17.alpha.-Ethynyl-19-nortestosterone active pharmaceutical ingredient (API) in detail. Different forms of 17.alpha.-Ethynyl-19-nortestosterone DMFs exist exist since differing nations have different regulations, such as 17.alpha.-Ethynyl-19-nortestosterone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 17.alpha.-Ethynyl-19-nortestosterone DMF submitted to regulatory agencies in the US is known as a USDMF. 17.alpha.-Ethynyl-19-nortestosterone USDMF includes data on 17.alpha.-Ethynyl-19-nortestosterone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 17.alpha.-Ethynyl-19-nortestosterone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The 17.alpha.-Ethynyl-19-nortestosterone Drug Master File in Japan (17.alpha.-Ethynyl-19-nortestosterone JDMF) empowers 17.alpha.-Ethynyl-19-nortestosterone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the 17.alpha.-Ethynyl-19-nortestosterone JDMF during the approval evaluation for pharmaceutical products. At the time of 17.alpha.-Ethynyl-19-nortestosterone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone suppliers with JDMF on PharmaCompass.
A 17.alpha.-Ethynyl-19-nortestosterone CEP of the European Pharmacopoeia monograph is often referred to as a 17.alpha.-Ethynyl-19-nortestosterone Certificate of Suitability (COS). The purpose of a 17.alpha.-Ethynyl-19-nortestosterone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of 17.alpha.-Ethynyl-19-nortestosterone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of 17.alpha.-Ethynyl-19-nortestosterone to their clients by showing that a 17.alpha.-Ethynyl-19-nortestosterone CEP has been issued for it. The manufacturer submits a 17.alpha.-Ethynyl-19-nortestosterone CEP (COS) as part of the market authorization procedure, and it takes on the role of a 17.alpha.-Ethynyl-19-nortestosterone CEP holder for the record. Additionally, the data presented in the 17.alpha.-Ethynyl-19-nortestosterone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the 17.alpha.-Ethynyl-19-nortestosterone DMF.
A 17.alpha.-Ethynyl-19-nortestosterone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. 17.alpha.-Ethynyl-19-nortestosterone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone suppliers with CEP (COS) on PharmaCompass.
A 17.alpha.-Ethynyl-19-nortestosterone written confirmation (17.alpha.-Ethynyl-19-nortestosterone WC) is an official document issued by a regulatory agency to a 17.alpha.-Ethynyl-19-nortestosterone manufacturer, verifying that the manufacturing facility of a 17.alpha.-Ethynyl-19-nortestosterone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting 17.alpha.-Ethynyl-19-nortestosterone APIs or 17.alpha.-Ethynyl-19-nortestosterone finished pharmaceutical products to another nation, regulatory agencies frequently require a 17.alpha.-Ethynyl-19-nortestosterone WC (written confirmation) as part of the regulatory process.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing 17.alpha.-Ethynyl-19-nortestosterone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for 17.alpha.-Ethynyl-19-nortestosterone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture 17.alpha.-Ethynyl-19-nortestosterone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain 17.alpha.-Ethynyl-19-nortestosterone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a 17.alpha.-Ethynyl-19-nortestosterone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of 17.alpha.-Ethynyl-19-nortestosterone suppliers with NDC on PharmaCompass.
17.alpha.-Ethynyl-19-nortestosterone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 17.alpha.-Ethynyl-19-nortestosterone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 17.alpha.-Ethynyl-19-nortestosterone GMP manufacturer or 17.alpha.-Ethynyl-19-nortestosterone GMP API supplier for your needs.
A 17.alpha.-Ethynyl-19-nortestosterone CoA (Certificate of Analysis) is a formal document that attests to 17.alpha.-Ethynyl-19-nortestosterone's compliance with 17.alpha.-Ethynyl-19-nortestosterone specifications and serves as a tool for batch-level quality control.
17.alpha.-Ethynyl-19-nortestosterone CoA mostly includes findings from lab analyses of a specific batch. For each 17.alpha.-Ethynyl-19-nortestosterone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
17.alpha.-Ethynyl-19-nortestosterone may be tested according to a variety of international standards, such as European Pharmacopoeia (17.alpha.-Ethynyl-19-nortestosterone EP), 17.alpha.-Ethynyl-19-nortestosterone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (17.alpha.-Ethynyl-19-nortestosterone USP).


LOOKING FOR A SUPPLIER?