
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
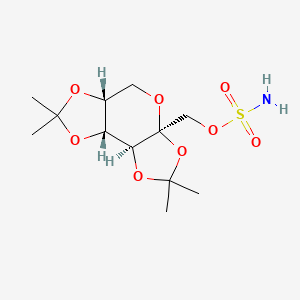

1. 2,3-4,5-bis-o-(1-methylethylidene)-beta-d-fructopyranose Sulfamate
2. Epitomax
3. Mcn 4853
4. Mcn-4853
5. Mcn4853
6. Topamax
7. Usl255
1. 97240-79-4
2. Topamax
3. Epitomax
4. Topamax Sprinkle
5. Topina
6. Mcn-4853
7. Tipiramate
8. Topiramatum
9. Topimax
10. Topomax
11. Tipiramato
12. Topiramato
13. Epitoma
14. Topamac
15. Mcn 4853
16. Rwj-17021
17. Rwj-17021-000
18. C12h21no8s
19. 2,3:4,5-bis-o-(1-methylethylidene)-beta-d-fructopyranose Sulfamate
20. Sincronil
21. Usl255
22. Qudexy
23. Usl-255
24. 2,3:4,5-di-o-isopropylidene-beta-d-fructopyranose Sulfamate
25. (-)-topiramate
26. [(3as,5ar,8ar,8bs)-2,2,7,7-tetramethyltetrahydro-3ah-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl Sulfamate
27. Chebi:63631
28. Tipiramate [french]
29. Tipiramato [spanish]
30. 0h73wjj391
31. ((3as,5ar,8ar,8bs)-2,2,7,7-tetramethyltetrahydro-3ah-bis([1,3]dioxolo)[4,5-b:4',5'-d]pyran-3a-yl)methyl Sulfamate
32. Topiramatum [inn-latin]
33. Topiramatum [latin]
34. Topiramato [inn-spanish]
35. Mfcd00865320
36. Dsstox_cid_3688
37. Dsstox_rid_77148
38. Dsstox_gsid_23688
39. ((3as,5ar,8ar,8bs)-2,2,7,7-tetramethyltetrahydro-3ah-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl)methyl Sulfamate
40. [(1r,2s,6s,9r)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0^{2,6}]dodecan-6-yl]methyl Sulfamate
41. Tpm
42. Topiragen
43. Topax
44. Trokendi Xr
45. Qudexy Xr
46. Smr000466325
47. Topiramate (tpm)
48. Tor
49. Topamax (tn)
50. Cas-97240-79-4
51. Rwj 17021
52. Brn 5988957
53. Unii-0h73wjj391
54. Hsdb 7531
55. Sulfamate 7
56. 3hku
57. 3lxe
58. Usl 255
59. Topiramate [usan:usp:inn:ban]
60. Topiramate Solution
61. Ncgc00095181-01
62. [(3as,5ar,8ar,8bs)-2,2,7,7-tetramethyl-5,5a,8a,8b-tetrahydrodi[1,3]dioxolo[5,4-b:5',3'-d]pyran-3a-yl]methyl Sulfamate
63. Topiramate- Bio-x
64. Trokendi Xr (tn)
65. Eprontia
66. Ks-1122
67. Topiramate [mi]
68. Topiramate [inn]
69. Topiramate [jan]
70. Spectrum2_001128
71. Topiramate [hsdb]
72. Topiramate [inci]
73. Topiramate [usan]
74. Beta-d-fructopyranose, 2,3:4,5-bis-o-(1-methylethylidene)-, Sulfamate
75. Topiramate [vandf]
76. Cbchromo1_000352
77. Topiramate [mart.]
78. Topiramate [usp-rs]
79. Topiramate [who-dd]
80. Bidd:pxr0127
81. Schembl34631
82. Bspbio_002306
83. Topiramate (jan/usp/inn)
84. Eprontia (liquid Formulation)
85. Mls000759431
86. Mls001424070
87. Bidd:gt0854
88. Spectrum1505801
89. Spbio_000995
90. Chembl220492
91. Gtpl6849
92. Dtxsid8023688
93. Topiramate [orange Book]
94. Bdbm10887
95. 2,3:4,5-di-o-isopropylidene-b-d-fructopyranose Sulfamate
96. Topiramate [ep Monograph]
97. Topiramate [usp Impurity]
98. Qsymia Component Topiramate
99. Hms1922h06
100. Hms2051l09
101. Hms2093d20
102. Hms2232h21
103. Hms3414c15
104. Hms3678c15
105. Hms3715i12
106. Hms3884c17
107. Pharmakon1600-01505801
108. Topiramate [usp Monograph]
109. [(3as,5ar,8ar,8bs)-2,2,7,7-tetramethyltetrahydro-3ah-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl Sulfamate (non-preferred Name)
110. Act09031
111. Albb-022457
112. Hy-b0122
113. Tox21_111472
114. Tox21_302401
115. Nsc759251
116. S1438
117. Zinc95616603
118. Topiramate Component Of Qsymia
119. .beta.-d-fructopyranose, 2,3:4,5-bis-o-(1-methylethylidene)-, Sulfamate
120. Akos000424547
121. Topiramate 1.0 Mg/ml In Acetonitrile
122. Topiramate, >=98% (hplc), Solid
123. Tox21_111472_1
124. Ccg-100940
125. Cs-1885
126. Db00273
127. Nc00190
128. Nsc 759251
129. Nsc-759251
130. Ncgc00178714-01
131. Ncgc00178714-04
132. Ncgc00178714-18
133. Ncgc00255221-01
134. Bt167048
135. Sbi-0206907.p001
136. Sw197570-3
137. C07502
138. D00537
139. F20536
140. Ab00639961-06
141. Ab00639961-08
142. Ab00639961_09
143. Ab00639961_10
144. 240t794
145. A900173
146. Q221174
147. Sr-01000759409
148. W-60376
149. Q-201845
150. Sr-01000759409-4
151. Z1522553470
152. Topiramate, United States Pharmacopeia (usp) Reference Standard
153. 2,3:4,5-bis-o-(1-methylethylidene)-?-d-fructopyranose Sulfamate
154. 2,3:4,5-di-o-isopropylidene-.beta.-d-fructopyranose Sulfamate
155. Topiramate, Pharmaceutical Secondary Standard; Certified Reference Material
156. .beta.-d-fructopyranose, 2,3:4,5-bis-o-(1-methylethylidene)-, 1-sulfamate
157. Topiramate Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
158. [(1r,2s,6s,9r)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.02,6]dodecan-6-yl]methyl Sulfamate
159. [(3as,5ar,8ar,8bs)-2,2,7,7-tetramethyl-5,5a,8a,8b-tetrahydrodi[1,3]dioxolo[4,5-a:5',3'-d]pyran-3a-yl]methyl Sulfamate
160. 2,3:4,5-bis-o-(1-methylethylidene)-beta-d-fructopyranose 1-sulfamate;2,3:4,5-di-o-isopropylidene-beta-d-fructopyranose Sulfamate; Topamax; Tracrium; Toiramate:
| Molecular Weight | 339.36 g/mol |
|---|---|
| Molecular Formula | C12H21NO8S |
| XLogP3 | -0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Exact Mass | 339.09878780 g/mol |
| Monoisotopic Mass | 339.09878780 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 556 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Topamax |
| PubMed Health | Topiramate (By mouth) |
| Drug Classes | Anticonvulsant, Central Nervous System Agent |
| Drug Label | Topiramate is a sulfamate-substituted monosaccharide. TOPAMAX (topiramate) Tablets are available as 25 mg, 50 mg, 100 mg, and 200 mg round tablets for oral administration. TOPAMAX (topiramate capsules) Sprinkle Capsules are available as 15 mg and... |
| Active Ingredient | Topiramate |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 200mg; 25mg; 15mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Topiramate |
| PubMed Health | Topiramate (By mouth) |
| Drug Classes | Anticonvulsant, Central Nervous System Agent |
| Drug Label | Topiramate is a sulfamate-substituted monosaccharide. Topiramate Tablets are available as 25 mg, 50 mg, 100 mg, and 200 mg round tablets for oral administration.Topiramate USP is a white crystalline powder with a bitter taste. Topiramate USP is most... |
| Active Ingredient | Topiramate |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 200mg; 25mg; 15mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Ranbaxy; Upsher Smith; Teva; Apotex; Accord Hlthcare; Sun Pharm Inds; Aurobindo Pharma; Torrent Pharms; Lupin; Invagen Pharms; Cipla; Hikma Pharms; Watson Labs; Glenmark Generics; Activis Totowa; Zydus Pharms Usa; Wockhardt Usa; Mylan; Unichem Labs |
| 3 of 6 | |
|---|---|
| Drug Name | Trokendi xr |
| Drug Label | Topiramate, USP, is a sulfamate-substituted monosaccharide. Trokendi XR (topiramate) extended-release capsules are available as 25 mg, 50 mg, 100 mg and 200 mg capsules for oral administration.Topiramate is a white to off-white powder. Topiramate... |
| Active Ingredient | Topiramate |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Supernus Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Topamax |
| PubMed Health | Topiramate (By mouth) |
| Drug Classes | Anticonvulsant, Central Nervous System Agent |
| Drug Label | Topiramate is a sulfamate-substituted monosaccharide. TOPAMAX (topiramate) Tablets are available as 25 mg, 50 mg, 100 mg, and 200 mg round tablets for oral administration. TOPAMAX (topiramate capsules) Sprinkle Capsules are available as 15 mg and... |
| Active Ingredient | Topiramate |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 200mg; 25mg; 15mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Topiramate |
| PubMed Health | Topiramate (By mouth) |
| Drug Classes | Anticonvulsant, Central Nervous System Agent |
| Drug Label | Topiramate is a sulfamate-substituted monosaccharide. Topiramate Tablets are available as 25 mg, 50 mg, 100 mg, and 200 mg round tablets for oral administration.Topiramate USP is a white crystalline powder with a bitter taste. Topiramate USP is most... |
| Active Ingredient | Topiramate |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 200mg; 25mg; 15mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Ranbaxy; Upsher Smith; Teva; Apotex; Accord Hlthcare; Sun Pharm Inds; Aurobindo Pharma; Torrent Pharms; Lupin; Invagen Pharms; Cipla; Hikma Pharms; Watson Labs; Glenmark Generics; Activis Totowa; Zydus Pharms Usa; Wockhardt Usa; Mylan; Unichem Labs |
| 6 of 6 | |
|---|---|
| Drug Name | Trokendi xr |
| Drug Label | Topiramate, USP, is a sulfamate-substituted monosaccharide. Trokendi XR (topiramate) extended-release capsules are available as 25 mg, 50 mg, 100 mg and 200 mg capsules for oral administration.Topiramate is a white to off-white powder. Topiramate... |
| Active Ingredient | Topiramate |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Supernus Pharms |
Topiramate is indicated as initial monotherapy in patients 10 years of age and older with partial onset or primary generalized tonic-clonic seizures. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2784
Topiramate is indicated for use in the adjunctive treatment of partial onset seizures in adults and pediatric patients ages 2 to 16 years. Topiramate is also indicated for use in the treatment of primary generalized tonic-clonic seizures in adults and in pediatric patients ages 2 to 16 years. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2784
Topiramate is indicated for use in the treatment of seizures associated with Lennox-Gastaut syndrome in patients 2 years of age and older. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
Topiramate is indicated for adults for the prophylaxis of migraine headache. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
The usefulness of topiramate in the acute treatment of migraine headache has not been studied. /NOT included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
Nervous system effects are the most frequently reported adverse effects of topiramate in adults and generally can be classified into 3 categories: cognitive-related dysfunction (e.g., confusion, psychomotor slowing, difficulty with concentration or attention, difficulty with memory, speech or language problems, particularly word-finding difficulties); psychiatric or behavioral disturbances (e.g., depression, mood problems); and somnolence or fatigue. Cognitive-related adverse effects frequently occur in isolation and often in association with a rapid titration rate and higher initial dosages. Although generally mild or moderate in severity, many of these cognitive-related adverse effects have resulted in discontinuance of topiramate therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2252
Psychiatric or behavioral disturbances (including rare cases of suicide attempts) appear to be dose-related in patients receiving topiramate for seizure disorders as well as for migraine prophylaxis. Somnolence and fatigue are the most commonly reported adverse effects in patients receiving topiramate for seizure disorders. In patients receiving topiramate as initial monotherapy for seizure disorders, the frequency of somnolence (but not fatigue) appears to be dose related. In patients receiving topiramate as adjunctive therapy for seizure disorders, the frequency of somnolence does not appear to be dose related; however, fatigue tends to occur with increasing frequency in patients receiving topiramate at dosages exceeding 400 mg daily. In patients receiving topiramate for migraine prophylaxis, somnolence and fatigue appear to be dose related and occur more frequently during the titration phase.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2252
Other common dose-related adverse nervous system effects of topiramate (at dosages of 200-1000 mg daily) include nervousness and anxiety. Frequently reported adverse nervous system effects that do not appear to be dose related include dizziness, ataxia, and paresthesia. Paresthesia occurred more frequently in patients receiving topiramate as initial monotherapy for management of seizure disorders or for migraine prophylaxis; however, in most instances, this adverse effect did not result in discontinuance of therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2253
Other common dose-related adverse effects of topiramate, in addition to adverse nervous system effects, include anorexia and weight loss. Frequently reported adverse effects that do not appear to be dose related include abnormal vision and diplopia.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2253
For more Drug Warnings (Complete) data for TOPIRAMATE (20 total), please visit the HSDB record page.
Topiramate is indicated for the following conditions: 1)Monotherapy for partial onset or primary generalized tonic-clonic seizures for patients 2 years of age and above 2)Adjunctive therapy for partial onset seizures or primary generalized tonic-clonic seizures for both adult and pediatric patients above 2 years old 3)Adjunctive therapy for seizures associated with Lennox-Gastaut syndrome in patients above 2 years of age 4)Prophylaxis of migraine in children 12 years of age and older and adults. Topiramate is also used off-label as an adjunct therapy for weight management and for mood disorders.
Topiramate prevents the occurrence of seizures and prevents migraine symptoms by reducing neural pathway excitability. It is important to note that this drug may cause metabolic acidosis, mood changes, suicidal thoughts and attempts, as well as kidney stones. When topiramate is combined with [valproic acid], it is known to cause hypothermia.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AX11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX11 - Topiramate
Absorption
After a 400mg dose in one clinical trial, topiramate reached maximal concentrations within 1.8-4.3 hours and ranged from 1.73-28.7 ug/mL. Food did not significantly affect the extent of absorption, despite delaying time to peak concentration. In patients with normal creatinine clearance, steady state concentrations are reached within 4 days. The bioavailability of topiramate in tablet form is about 80% compared to a topiramate solution.
Route of Elimination
Topiramate is mainly eliminated through the kidneys. About 70-80% of the eliminated dose is found unchanged in the urine.
Volume of Distribution
The mean apparent volume of distribution of topiramate ranges from 0.6-0.8 L/kg when doses of 100mg to 1200mg are given. Topiramate readily crosses the blood-brain barrier.
Clearance
The mean oral plasma clearance of topiramate ranges from 22-36 mL/min while the renal clearance is 17-18 mL/min, according to one pharmacokinetic study. The FDA label for topiramate indicates a similar oral plasma clearance of approximately 20 to 30 mL/min in adults.
Absorption /of topiramate is/ rapid. The bioavailability of the tablet dosage form is about 80% as compared with that from a solution. Food does not effect the bioavailability of topiramate.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
Protein binding /of topiramate is/ low ( 13 to 17% over the concentration range of 1 to 250 ug per mL).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
Time to peak concentration /is/ approximately 2 hours following administration of a 400 mg oral dose. In patients with normal renal function, steady state is reached in about 4 days. The pharmacokinetics or topiramate are linear, with dose proportional increases in the plasma concentration over the range of 200 to 800 mg a day.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
Distribution of topiramate into human milk has not been evaluated in controlled studies; however, limited data indicate that the drug may distribute extensively into milk in humans.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2253
For more Absorption, Distribution and Excretion (Complete) data for TOPIRAMATE (11 total), please visit the HSDB record page.
The metabolites of topiramate are not known to be active. The metabolism of topiramate is characterized by reactions of glucuronidation, hydroxylation and hydrolysis that lead to the production of six minor metabolites. Some of topiramate's metabolites include 2,3-desisopropylidene topiramate, 4,5-desisopropylidene topiramate, 9-hydroxy topiramate, and 10-hydroxy topiramate.
Topiramate is not extensively metabolized. Six minor metabolites (formed by hydroxylation, hydrolysis, and glucuronidation) have been identified in humans, with none constituting more than 5% of an administered dose.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
The metabolism and excretion of 2,3:4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate (TOPAMAX, topiramate, TPM) have been investigated in animals and humans. Radiolabeled [14C] TPM was orally administered to mice, rats, rabbits, dogs and humans. Plasma, urine and fecal samples were collected and analyzed. TPM and a total of 12 metabolites were isolated and identified in these samples. Metabolites were formed by hydroxylation at the 7- or 8-methyl of an isopropylidene of TPM followed by rearrangement, hydroxylation at the 10-methyl of the other isopropylidene, hydrolysis at the 2,3-O-isopropylidene, hydrolysis at the 4,5-O-isopropylidene, cleavage at the sulfamate group, glucuronide conjugation and sulfate conjugation. A large percentage of unchanged TPM was recovered in animal and human urine. The most dominant metabolite of TPM in mice, male rats, rabbits and dogs appeared to be formed by the hydrolysis of the 2,3-O-isopropylidene group.
PMID:16250251 Caldwell G et al; Eur J Drug Metab Pharmacokinet 30 (3): 151-64 (2005)
The elimination half-life is reported to be in the range of 19-23 hours. If topiramate is given with enzyme-inducers, the half-life can be reduced to 12-15 hours because of increased metabolism.
21 hours (mean) after single or multiple dosing.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
A seizure is an abnormal and unregulated electrical discharge occurring in the brain. This leads to transient interruption in brain function, manifested by reduced alertness, abnormal sensations, and focal involuntary movements or convulsions. Several types of seizures exist, with common types including tonic-clonic seizures and partial onset seizures. The exact mechanisms by which topiramate exerts pharmacological actions on seizures and migraines are currently not fully characterized. Several properties of this drug, however, are likely to contribute to its therapeutic effects. Topiramate has been observed to exert actions on voltage-dependent sodium channels, GABA receptors, and glutamate receptors. Topiramate stimulates GABA-A receptor activity at brain non-benzodiazepine receptor sites and reduces glutamate activity at both AMPA and kainate receptors. Normally, GABA-A receptors are inhibitory and glutaminergic receptors are stimulatory for neuronal activity. By increasing GABA activity and inhibiting glutamate activity, topiramate blocks neuronal excitability, preventing seizures and migraines. Additionally, it blocks the voltage-dependent sodium channels, further blocking seizure activity. Topiramate has been shown to inhibit various carbonic anhydrase isozymes, but the clinical significance of this is unknown at this time.
The precise mechanism of action is unknown. Electrophysiological and biochemical studies on cultured neurons demonstrated that topiramate blocks the action potentials elicited repetitively by a sustained depolarization of the neurons in a time dependent manner; this effect suggests a state-dependent sodium channel blocking action. Also topiramate increases the frequency at which gamma-aminobutyric acid (GABA) activates GABA-A receptors, thereby enhancing GABA-induced influx of chloride ions into neurons. Thus, it appears that topiramate exerts it effects by potentiation of the activity of the inhibitory neurotransmitter, GABA. In addition, topiramate antagonizes the ability of kainate to activate the kainate/AMPA (alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; non-NMDA) subtype of excitatory amino acid (glutamate) receptor, but has no apparent effect on the activity of N-methyl-D-aspartate (NMDA) at the NMDA receptor subtype. These effects of topiramate are concentration-dependent within the range of 1 to 200 micromoles.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2785
Although the precise mechanism of action of topiramate is unknown, data from electrophysiologic and biochemical studies have revealed 4 properties that may contribute to the drug's efficacy for seizure disorders and migraine prophylaxis. At pharmacologically relevant concentrations, topiramate blocks voltage-dependent sodium channels; augments the activity of gamma-aminobutyric acid (GABA) at some subtypes of the GABA-A receptor; antagonizes the AMPA/kainate subtype of the glutamate receptor; and inhibits carbonic anhydrase (particularly CA-II and CA-IV isoenzymes). In general, anticonvulsant drugs are thought to act by one or more of the following mechanisms: modulating voltage-dependent ion (e.g., sodium) channels involved in action potential propagation or burst generation, enhancement of GABA inhibitory activity, and/or inhibition of excitatory amino acid neurotransmitter (e.g., glutamate, aspartate) activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2254
Topiramate exhibits effects on cultured neurons similar to those observed with phenytoin and carbamazepine, and such effects are suggestive of an inactive state-dependent block of voltage-dependent sodium channels. Topiramate reduces the duration of epileptiform bursts of neuronal firing and decreases the number of action potentials in studies of cultured rat hippocampal neurons with spontaneous epileptiform burst activity. Topiramate also decreases the frequency of action potentials elicited by depolarizing electric current in cultured rat hippocampal neurons. Depolarization and firing of an action potential results from the rapid inflow of sodium ions through voltage-dependent sodium channels in the neuronal cell membrane. After firing, a neuron enters a period of inactivation during which it is unable to fire again even if the sodium channel is open. A slow action potential firing rate allows the neuron sufficient time to recover from inactivation, and the normal period of inactivation has a minimal effect on low-frequency firing. During a partial seizure, neurons characteristically undergo high-frequency depolarization and firing of action potentials which is uncommon during normal physiologic neuronal activity. Some anticonvulsant drugs (e.g., phenytoin, carbamazepine) preferentially bind to voltage-dependent sodium channels during their inactivated state, slow the rate of recovery of sodium channels from their period of inactivation, and limit the ability of the neuron to depolarize and fire at high frequencies.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2254
Topiramate enhances the activity of the inhibitory neurotransmitter GABA at a nonbenzodiazepine site on GABA-A receptors. Activation of the postsynaptic GABA-A receptor by GABA causes inhibition by increasing the inward flow of chloride ions, resulting in hyperpolarization of the postsynaptic cell; in chloride ion-depleted murine cerebellar granule cells, therapeutic concentrations of topiramate (in combination with GABA) enhance GABA-evoked inward flux of chloride ions in a concentration-dependent manner. Benzodiazepines act at GABA-A receptors to enhance GABA-evoked inward flow of chloride ions, but the benzodiazepine antagonist flumazenil does not appear to inhibit topiramate enhancement of GABA-evoked currents in GABA-A cortical neuronal receptors. Topiramate also does not appear to increase duration of chloride ion channel opening. Therefore, topiramate may potentiate GABA-A-evoked chloride ion flux by a mechanism other than GABA-A-receptor modulation.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2254
For more Mechanism of Action (Complete) data for TOPIRAMATE (8 total), please visit the HSDB record page.
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
GDUFA
DMF Review : Complete
Rev. Date : 2015-04-03
Pay. Date : 2013-05-21
DMF Number : 20581
Submission : 2007-06-06
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2016-11-10
Pay. Date : 2016-09-22
DMF Number : 17729
Submission : 2004-10-06
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16405
Submission : 2003-01-30
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10978
Submission : 1994-07-08
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15693
Submission : 2001-10-31
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-09-04
Pay. Date : 2013-08-26
DMF Number : 18346
Submission : 2005-05-12
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 10919
Submission : 1994-05-27
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17035
Submission : 2003-12-16
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-09-25
Pay. Date : 2023-09-20
DMF Number : 18172
Submission : 2005-03-04
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2020-140 - Rev 04
Status : Valid
Issue Date : 2025-03-21
Type : Chemical
Substance Number : 2616

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2020-130 - Rev 01
Status : Valid
Issue Date : 2023-03-06
Type : Chemical
Substance Number : 2616

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2020-201 - Rev 01
Status : Valid
Issue Date : 2022-03-02
Type : Chemical
Substance Number : 2616

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2021-419 - Rev 01
Status : Valid
Issue Date : 2024-10-09
Type : Chemical
Substance Number : 2616

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Date of Issue : 2022-08-25
Valid Till : 2025-07-02
Written Confirmation Number : WC-0156
Address of the Firm : 61-B, Bommasandra Industrial Area, Bengaluru-560 099, India
Date of Issue : 2022-06-07
Valid Till : 2025-06-25
Written Confirmation Number : WC-0057n
Address of the Firm : Plot No. 3109, GIDC, Industrial Estate, Ankleshwar-393 002, Bharuch, Gujarat

Date of Issue : 2022-07-15
Valid Till : 2025-07-02
Written Confirmation Number : WC-0120
Address of the Firm : Unit-1, Sy. No. 388 & 389, Borpatla (V), Hatnoora (M), Medak (Dist).502 296 A.P....

Date of Issue : 2022-06-20
Valid Till : 2025-08-08
Written Confirmation Number : WC-0113
Address of the Firm : Old Madras Road, Virgo Nagar Post, Bengaluru - 560049

Date of Issue : 2019-08-09
Valid Till : 2025-08-08
Written Confirmation Number : WC-0066
Address of the Firm : Plot No. 1, Hetero SEZ Infrastructure Ltd., Narasapuram, Visakhapatnam-531 081, ...

Date of Issue : 2022-07-04
Valid Till : 2025-07-03
Written Confirmation Number : WC-0040
Address of the Firm : Unit-I, Survey No.213, 214 & 255, Bonthapally Village, Jinnaram Mandal, Medak ...

Date of Issue : 2020-04-15
Valid Till : 2023-05-19
Written Confirmation Number : WC-0281new2
Address of the Firm : Sy. No. 224/A, Bibinagar(V) & (M), Bhongir, Nalgonda District, Andhra Pradesh

Date of Issue : 2018-04-05
Valid Till : 2021-04-05
Written Confirmation Number : WC-316
Address of the Firm : Plot No.50-A,B,G & H, 64-A,B, C&D, 65-A,B,C&D, 66-A & B, 67-A & B, IDA, Kondapal...

Date of Issue : 2022-08-08
Valid Till : 2025-07-14
Written Confirmation Number : WC-0168
Address of the Firm : Plot No. 24/2, & 25, Phase -IV, GIDC, Industrial Zone, At & Post - Panoli, Dist....

Date of Issue : 2024-04-23
Valid Till : 2027-04-22
Written Confirmation Number : WC-0170
Address of the Firm : Plot No. 41, 42/2, 42/1/b, 43, 40, 59, 58, GIDC, Vapi, Dist-Valsad, Gujarat, Ind...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0492
Start Marketing Date : 1996-12-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0410
Start Marketing Date : 1996-12-24
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 66039-806
Start Marketing Date : 2004-10-06
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 66039-806
Start Marketing Date : 2004-10-06
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 53104-7532
Start Marketing Date : 2016-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (40kg/40kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65977-0008
Start Marketing Date : 1996-12-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 50379-0007
Start Marketing Date : 2012-06-21
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65129-1024
Start Marketing Date : 2002-12-26
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 69056-501
Start Marketing Date : 2024-04-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 17205-096
Start Marketing Date : 1996-12-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
About the Company : Bal Pharma is a leading Indian pharmaceutical company with 30+ years of experience, specializing in prescription drugs, generic and OTC products, intravenous infusions, and bulk ac...
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
About the Company : Polpharma API, part of a leading Polish pharmaceutical group, has over 70 years of experience in process development and cGMP manufacturing. We offer end-to-end solutions, from API...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
About the Company : BIOTECHNICA DWC LLC has carved a niche for itself in providing value added compliance, regulatory qualification, project management and GDP guidance services to pharma companies al...

About the Company : Fleming Laboratories Limited is in the business of manufacturing and supply of high-quality generic Active Pharmaceutical Ingredients (APIs) to the global Pharmaceutical Industry. ...

About the Company : Guangzhou Tosun Pharmaceutical was founded in 1999, which mainly focuses on importation & exportation of Active Pharmaceutical Ingrediants, Chemical Raw Materials, Intermediate, Ex...

About the Company : Hermes Chemical Company Pvt Ltd is a chemicals company established in 2001 and headquartered in Hyderabad. It is specialized in developing, manufacturing, and selling complex molec...

About the Company : Hetero is a research based global pharmaceutical company focused on development, manufacturing and marketing of Active Pharmaceutical Ingredients (APIs), Intermediate Chemicals & F...

About the Company : ScinoPharm Taiwan Ltd. is a leading process R&D and API manufacturing service provider to the global pharmaceutical industry. With cGMP production facilities, ScinoPharm offers a w...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : Microlose (Lactose Monohydrate & Microcrystalline Cellulose) is used as a diluent in oral dosage forms such as tablets.
Pharmacopoeia Ref : DMF, EXCiPAT, KOSHER, HALAL, W...
Technical Specs : Lactose Monohydrate – 40%, Microcrystalline cellulose – 60%
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Oral
Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : ProBlend (SMCC) is a co-processed excipient consists of microcrystalline cellulose & colloidal silicon dioxide, used as a diluent & binder in OSDs.
Pharmacopoeia Ref : USP-NF, DMF, EXCiPAT, KOSHER, ...
Technical Specs : NA
Ingredient(s) : Silicified Microcrystalline Cellulose
Dosage Form : Capsule
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : Sallyso (Croscarmellose Sodium) is a cross-linked polymer of carboxymethylcellulose sodium, used as a superdisintegrant in tablets and capsules.
Pharmacopoeia Ref : USP-NF, BP, IP, EP, DMF, EXCiP...
Technical Specs : Sallyso 0.5 to 3.0%
Ingredient(s) : Croscarmellose Sodium
Dosage Form : Capsule
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : Solvostar (Sodium Starch Glycolate) is used as a superdisintegrant in oral solid dosage forms such as tablets and capsules.
Pharmacopoeia Ref : USP-NF, BP, IP, EP, DMF, EXCiP...
Technical Specs : Solvostar 2% to 8%
Ingredient(s) : Sodium Starch Glycolate
Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : Starlose P40 (Starch & Lactose Monohydrate) is used as a diluent in oral dosage forms such as tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Not Available
Application : Controlled & Modified Release
Excipient Details : Solubilization, dispersion, crystallization inhibition, instant release matrices & spray drying
Dosage Form : Tablet
Grade : Not Available
Application : Solubilizers
Excipient Details : Solubilization, dispersion, crystallization inhibition, instant release matrices & spray drying
Pharmacopoeia Ref : Ph. Eur., USP, JP: Povidone
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Capsule
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Tablet binding, thickener, stabilizers of suspensions, reduces sedimentation, crystallization inhibition.
Pharmacopoeia Ref : Ph. Eur., USP, JP: Povidone
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Tablet
Grade : Not Available
Application : Granulation
Excipient Details : For non-erodible matrices using direct compression, Controlled release matrix. Matrix former in transdermal patches and topical films.
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP-JPE: 80 %...
Technical Specs : Not Available
Ingredient(s) : Povidone
Dosage Form : Tablet
Grade : Not Available
Application : Granulation
Excipient Details : Wetting agent, reduces disintegration time, Ionic solubilizer, high HLB anionic emulsifier for semi-solids and foams.
Pharmacopoeia Ref : Ph. Eur.: Sodium Laurilsulfate...
Technical Specs : Not Available
Ingredient(s) : Sodium Lauryl Sulfate
Dosage Form : Capsule
Grade : Not Available
Dosage Form : Tablet
Grade : Not Available
Application : Film Formers & Plasticizers
Excipient Details : Liquid plasticizer with high ADI, hydrophilic solvent & humectant in emulsions, skin penetration enhancer in topical formulaitons.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Propylene Glycol
Dosage Form : Orodispersible Tablet
Grade : Not Available
Application : Chewable & Orodispersible Aids
Excipient Details : Ludiflash is a ready-to-use orally disintegrating tablet (ODT) solution with superior mouthfeel.
Pharmacopoeia Ref : Ph. Eur., USP, JP: 90 % mannit...
Technical Specs : Not Available
Ingredient(s) : Crospovidone
Dosage Form : Tablet
Grade : Not Available
Application : Direct Compression
Excipient Details : Ready-to-use direct compression solution for tablets.
Dosage Form : Tablet
Grade : Not Available
Application : Direct Compression
Excipient Details : Ready-to-use direct compression solution for lozenges, chewables and effervescent tablets.
Pharmacopoeia Ref : Ph. Eur., USP/NF and J.P
Technical Specs : Not Available
Ingredient(s) : Povidone
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
71
PharmaCompass offers a list of Topiramate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Topiramate manufacturer or Topiramate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Topiramate manufacturer or Topiramate supplier.
PharmaCompass also assists you with knowing the Topiramate API Price utilized in the formulation of products. Topiramate API Price is not always fixed or binding as the Topiramate Price is obtained through a variety of data sources. The Topiramate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate, including repackagers and relabelers. The FDA regulates [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate supplier is an individual or a company that provides [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate active pharmaceutical ingredient (API) or [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate finished formulations upon request. The [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers may include [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate API manufacturers, exporters, distributors and traders.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate DMF (Drug Master File) is a document detailing the whole manufacturing process of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate active pharmaceutical ingredient (API) in detail. Different forms of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate DMFs exist exist since differing nations have different regulations, such as [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate DMF submitted to regulatory agencies in the US is known as a USDMF. [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate USDMF includes data on [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate Drug Master File in Japan ([(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate JDMF) empowers [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate JDMF during the approval evaluation for pharmaceutical products. At the time of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate Drug Master File in Korea ([(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate. The MFDS reviews the [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate KDMF as part of the drug registration process and uses the information provided in the [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate KDMF to evaluate the safety and efficacy of the drug.
After submitting a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with KDMF on PharmaCompass.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP of the European Pharmacopoeia monograph is often referred to as a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate Certificate of Suitability (COS). The purpose of a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate to their clients by showing that a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP has been issued for it. The manufacturer submits a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP (COS) as part of the market authorization procedure, and it takes on the role of a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP holder for the record. Additionally, the data presented in the [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate DMF.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with CEP (COS) on PharmaCompass.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate written confirmation ([(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate WC) is an official document issued by a regulatory agency to a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate manufacturer, verifying that the manufacturing facility of a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate APIs or [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate finished pharmaceutical products to another nation, regulatory agencies frequently require a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate WC (written confirmation) as part of the regulatory process.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate suppliers with NDC on PharmaCompass.
[(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate GMP manufacturer or [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate GMP API supplier for your needs.
A [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CoA (Certificate of Analysis) is a formal document that attests to [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate's compliance with [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate specifications and serves as a tool for batch-level quality control.
[(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CoA mostly includes findings from lab analyses of a specific batch. For each [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
[(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate may be tested according to a variety of international standards, such as European Pharmacopoeia ([(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate EP), [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate JP (Japanese Pharmacopeia) and the US Pharmacopoeia ([(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0?,?]dodecan-6-yl]methyl sulfamate USP).
