
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Finished Drug Prices
NA
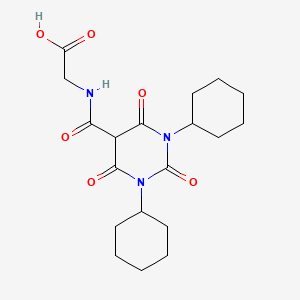

1. 2-(1,3-dicyclohexyl-6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamido)acetic Acid
2. Gsk1278863
1. 960539-70-2
2. Gsk1278863
3. Gsk-1278863
4. N-((1,3-dicyclohexylhexahydro-2,4,6-trioxopyrimidin-5-yl)carbonyl)glycine
5. Gsk 1278863
6. Jvr38zm64b
7. 2-[(1,3-dicyclohexyl-2,4,6-trioxo-1,3-diazinan-5-yl)formamido]acetic Acid
8. Glycine, N-((1,3-dicyclohexylhexahydro-2,4,6-trioxo-5-pyrimidinyl)carbonyl)-
9. Duvroq
10. (1,3-dicyclohexyl-2,4,6-trioxohexahydropyrimidine-5-carbonyl)glycine
11. 2-[(1,3-dicyclohexyl-2,4,6-trioxo-1,3-diazinane-5-carbonyl)amino]acetic Acid
12. Daprodustat [usan:inn]
13. Unii-jvr38zm64b
14. Duvroq (tn)
15. Daprodustat [inn]
16. Daprodustat [jan]
17. Daprodustat [usan]
18. Daprodustat [who-dd]
19. Daprodustat; Gsk1278863
20. Daprodustat (jan/usan/inn)
21. Gtpl8455
22. Daprodustat (gsk1278863)
23. Chembl3544988
24. Schembl21725048
25. Dtxsid501337360
26. Amy27907
27. Bcp16766
28. Ex-a1121
29. Mfcd29924726
30. S8171
31. Akos027439964
32. Zinc231226004
33. Ccg-268574
34. Cs-5453
35. Db11682
36. Sb19761
37. Ac-30915
38. Bg166670
39. Hy-17608
40. Db-096637
41. J3.560.573h
42. D10874
43. A902761
44. Q27076986
45. Daprodustat;(1,3-dicyclohexyl-2,4,6-trioxohexahydropyrimidine-5-carbonyl)glycine
| Molecular Weight | 393.4 g/mol |
|---|---|
| Molecular Formula | C19H27N3O6 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 393.18998559 g/mol |
| Monoisotopic Mass | 393.18998559 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 627 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of anaemia due to chronic disorders
B - Blood and blood forming organs
B03 - Antianemic preparations
B03X - Other antianemic preparations
B03XA - Other antianemic preparations
B03XA07 - Daprodustat
About the Company : Established in May 2012, Shandong Loncom Pharmaceutical operates as a fully owned subsidiary of Shandong Bestcomm Pharmaceutical Co., Ltd. Situated in the Qihe Economic Development...
About the Company : Inke S.A., is focused since 1980 in the development and manufacture of the highest quality Active Pharmaceutical Ingredients (APIs) with complex synthesis processes for diverse the...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
About the Company : Ami Lifesciences, established in 2006, is one of the fastest growing API manufacturing companies in India. Specializing in cardiovascular, anti-diabetic, CNS, and respiratory thera...
 Jinan Tantu Chemicals offers customized R&D services & production of small molecule APIs & pharmaceutical intermediates.
Jinan Tantu Chemicals offers customized R&D services & production of small molecule APIs & pharmaceutical intermediates.
About the Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our core services include c...
 Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
Sichuan Elixir Pharmaceutical, a manufacturer of small molecule APIs & a CMO/CDMO service provider for anti-tumor characteristic APIs..
About the Company : Specializing in natural & oncology APIs, we establish R&D and production platforms for new salt, crystal form & synthetic biology research. We cooperate with clients for IND, NDA, ...
About the Company : Hebi Xinhe Pharmaceutical Co., Ltd. is a subsidiary of Tianjin Zhennuo Pharmaceutical Group Co., Ltd., with a registered capital of CNY 100 million. Located in Jijiashan Industrial...

About the Company : Micro Labs Limited is a diversified healthcare company with cutting-edge R&D, advanced manufacturing facilities, and a strong distribution network. It ranks among India's top pharm...

About the Company : Established in the early 90’s SCL offers a wide range of Active Pharmaceutical Ingredients and Intermediates to its customers worldwide. SCL’s products are exported to Europe, ...

About the Company : Shanvr Life Sciences Pvt Ltd is a pharmaceutical product development company with a strong emphasis on research. Our approach centers around the creation of specialized generic, on...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?