
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
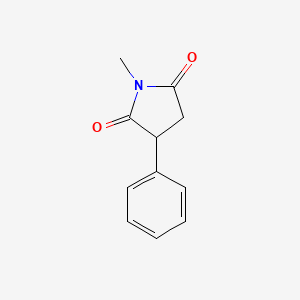

1. Phensuccimide
2. Phensuximide, (+-)-isomer
1. 86-34-0
2. Succitimal
3. Milontin
4. Lifene
5. Epimid
6. 1-methyl-3-phenylpyrrolidine-2,5-dione
7. Phensuximid
8. Phenylsuximide
9. Milonton
10. Mirontin
11. 1-methyl-3-phenyl-2,5-pyrrolidinedione
12. Phensuximidum
13. Succinimide, N-methyl-2-phenyl-
14. Racemic Phensuximide
15. N-methyl-2-phenylsuccinimide
16. 1-methyl-3-phenylsuccinimide
17. Pm 334
18. 1-methyl-3-phenylpyrrolidin-2,5-dione
19. Ai3-20372
20. 2,5-pyrrolidinedione, 1-methyl-3-phenyl-
21. Nsc-760079
22. 6wvl9c355g
23. Chebi:8079
24. Phensuccimide
25. Fenosuccimide
26. N-methyl-.alpha.-phenylsuccinimide
27. Epimid; Lifene; Milontin; Milonton
28. Pm-334
29. Fensuccimide
30. Methylphenylsuccinimide
31. Fensuccimide [dcit]
32. Dsstox_cid_3460
33. Dsstox_rid_77035
34. Dsstox_gsid_23460
35. Fensuximida [inn-spanish]
36. Phensuximidum [inn-latin]
37. Fensuximida
38. N-methyl-alpha-phenylsuccinimide
39. Rs-phensuximide
40. Milontin (tn)
41. (+-)-n-methyl-2-phenylsuccinimide
42. Einecs 201-664-6
43. Brn 0155329
44. Unii-6wvl9c355g
45. Lifen
46. Phensuximide [usp:inn:ban]
47. Cas-86-34-0
48. Ncgc00016339-01
49. (+/-)-n-methyl-2-phenylsuccinimide
50. Prestwick0_001061
51. Prestwick1_001061
52. Prestwick2_001061
53. Prestwick3_001061
54. Phensuximide (usp/inn)
55. Phensuximide [mi]
56. Phensuximide [inn]
57. Chembl797
58. Phensuximide [vandf]
59. Schembl35333
60. Bspbio_001042
61. Phensuximide [mart.]
62. 5-21-11-00188 (beilstein Handbook Reference)
63. Mls002154134
64. (+/-)-phensuximide
65. N-methyl-2-phenyl-succinimide
66. Phensuximide [usp-rs]
67. Phensuximide [who-dd]
68. 2,5-pyrrolidinedione, 1-methyl-3-phenyl-, (+-)-
69. Spbio_002961
70. Bpbio1_001148
71. Gtpl7612
72. Dtxsid4023460
73. Hms1571e04
74. Hms2098e04
75. Hms2235h10
76. Hms3264e08
77. Hms3369l21
78. Hms3715e04
79. Hms3887e05
80. Pharmakon1600-01505458
81. Phensuximide [orange Book]
82. Phensuximide [usp Impurity]
83. Hy-b1730
84. N-methyl-3-phenylsuccinimide
85. Tox21_110382
86. Ac7001
87. Mfcd00072136
88. Nsc760079
89. Akos006230701
90. Tox21_110382_1
91. Ccg-213454
92. Db00832
93. Nsc 760079
94. (.+/-.)-n-methyl-2-phenylsuccinimide
95. N-methyl-3-phenylpyrrolidinedione
96. Ncgc00179333-01
97. Ncgc00179333-03
98. Ac-15962
99. Ai320372
100. Smr001233441
101. Sy251116
102. 1-methyl-3-phenyl-2,5-pyrrolidinedione #
103. Ai3 20372
104. Ab00514025
105. Cs-0013738
106. C07437
107. D00508
108. Ab00514025_07
109. 086p340
110. Q999805
111. Sr-01000842158
112. Sr-01000842158-2
113. Brd-a18043272-001-03-2
114. 2,5-pyrrolidinedione, 1-methyl-3-phenyl-, (+/-)-
115. 34367-67-4
| Molecular Weight | 189.21 g/mol |
|---|---|
| Molecular Formula | C11H11NO2 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 189.078978594 g/mol |
| Monoisotopic Mass | 189.078978594 g/mol |
| Topological Polar Surface Area | 37.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 256 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of epilepsy.
Phensuximide suppresses the paroxysmal three cycle per second spike and wave EEG pattern associated with lapses of consciousness in absence (petit mal) seizures. The frequency of attacks is reduced by depression of nerve transmission in the motor cortex.
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AD - Succinimide derivatives
N03AD02 - Phensuximide
Absorption
Rapid and complete.
Hepatic.
Phensuximide's mechanism of action not understood, but may act in inhibitory neuronal systems that are important in the generation of the three per second rhythm. It's effects may be related to its ability to inhibit depolarization-induced accumulation of cyclic AMP and cyclic GMP in brain tissue.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?