
Synopsis
Synopsis
0
JDMF
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
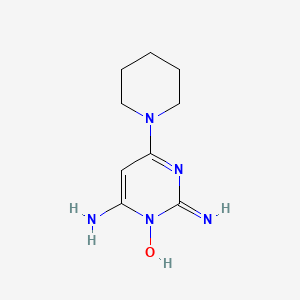

1. Loniten
2. Regaine
3. Rogaine
4. U 10858
1. 38304-91-5
2. Rogaine
3. Loniten
4. Minoximen
5. Regaine
6. Theroxidil
7. Alopexil
8. Alostil
9. Tricoxidil
10. Lonolox
11. Normoxidil
12. Prexidil
13. Minodyl
14. Pierminox
15. Riup
16. Mintop
17. 6-(1-piperidinyl)-2,4-pyrimidinediamine 3-oxide
18. 2,4-pyrimidinediamine, 6-(1-piperidinyl)-, 3-oxide
19. U-10858
20. C9h15n5o
21. 3-hydroxy-2-imino-6-piperidin-1-ylpyrimidin-4-amine
22. Chebi:6942
23. 6-(piperidin-1-yl)pyrimidine-2,4-diamine 3-oxide
24. U-10,858
25. 6-amino-1,2-dihydro-1-hydroxy-2-imino-4-piperidinopyrimidine
26. Nsc-757106
27. Mls000028566
28. Minossidile [italian]
29. Minoxidilum [inn-latin]
30. Ncgc00015673-08
31. Minoxidilum
32. Smr000058963
33. 6-amino-2-imino-4-(piperidin-1-yl)-1,2-dihydropyrimidin-1-ol
34. Cas-38304-91-5
35. Apo-gain
36. U10858
37. Dsstox_cid_20685
38. Dsstox_rid_79541
39. 6-piperidin-1-ylpyrimidine-2,4-diamine 3-oxide
40. Dsstox_gsid_40685
41. 6-(1-piperidinyl)-2,4-pyrimidinediamine-3-oxide
42. 2,6-diamino-4-(piperidin-1-yl)pyrimidin-1-ium-1-olate
43. Neoxidil
44. Avacor And Mintop
45. Smr000326812
46. Loniten (tn)
47. Rogaine (tn)
48. Riup (tn)
49. 2,4-diamino-6-piperidinopyrimidine 3-oxide
50. Sr-01000075331
51. Sr-05000001479
52. Mfcd00063409
53. 3-oxido-6-piperidin-1-ylpyrimidin-3-ium-2,4-diamine
54. Kopdil
55. Regaine For Men
56. Minoxidil,(s)
57. Regaine For Women
58. Prestwick_521
59. 3-hydroxy-2-imino-6-(1-piperidyl)pyrimidin-4-amine
60. Tm-160
61. Men''''s Rogaine
62. Women''''s Rogaine
63. 6-amino-2-imino-4-(piperidin-1-yl)pyrimidin-1(2h)-ol
64. Rogaine Extra Strength
65. Pyrimidin-1(2h)-ol
66. Spectrum_000969
67. Tocris-0583
68. Minoxidil [inn]
69. Minoxidil [jan]
70. Minoxidil [mi]
71. Minoxidil Extra Strength
72. Minoxidil [hsdb]
73. Minoxidil [inci]
74. Minoxidil [usan]
75. Regid855572
76. Opera_id_1150
77. Prestwick0_000020
78. Prestwick1_000020
79. Prestwick2_000020
80. Prestwick3_000020
81. Spectrum2_001053
82. Spectrum3_000509
83. Spectrum4_000063
84. Spectrum5_001299
85. Lopac-m-4145
86. Minoxidil [vandf]
87. M1389
88. Chembl802
89. Minoxidil [mart.]
90. M 4145
91. Minoxidil [usp-rs]
92. Minoxidil [who-dd]
93. Minoxidil (u-10858)
94. Minoxidil (jan/usp/inn)
95. Cbiol_001798
96. Lopac0_000786
97. Schembl29698
98. Bspbio_000059
99. Bspbio_001385
100. Bspbio_002037
101. Kbiogr_000105
102. Kbiogr_000585
103. Kbioss_000105
104. Kbioss_001449
105. 16317-69-4
106. Mls000859953
107. Mls001077294
108. Divk1c_000160
109. Schembl232565
110. Spectrum1500415
111. Spbio_001006
112. Spbio_001980
113. Bpbio1_000065
114. Chembl609587
115. Gtpl4254
116. Minoxidil, >=99% (tlc)
117. Sgcut00112
118. Zinc1735
119. Minoxidil [orange Book]
120. Chembl1372483
121. Dtxsid9040685
122. Minoxidil [ep Monograph]
123. Bcbcmap01_000193
124. Bdbm81463
125. Chebi:92128
126. Hms500h22
127. Kbio1_000160
128. Kbio2_000105
129. Kbio2_001449
130. Kbio2_002673
131. Kbio2_004017
132. Kbio2_005241
133. Kbio2_006585
134. Kbio3_000209
135. Kbio3_000210
136. Kbio3_001537
137. Minoxidil [usp Monograph]
138. Ninds_000160
139. Bcpp000162
140. Bio1_000084
141. Bio1_000573
142. Bio1_001062
143. Bio2_000105
144. Bio2_000585
145. Hms1361f07
146. Hms1568c21
147. Hms1791f07
148. Hms1920p03
149. Hms1989f07
150. Hms2089l08
151. Hms2091f20
152. Hms2095c21
153. Hms2233e04
154. Hms2235n21
155. Hms3259p21
156. Hms3262m14
157. Hms3266o06
158. Hms3371e15
159. Hms3372m19
160. Hms3402f07
161. Hms3411g16
162. Hms3675g16
163. Hms3712c21
164. Pharmakon1600-01500415
165. Act04612
166. Bcp01409
167. Cas_4201
168. Hy-b0112
169. Nsc_4201
170. To_000070
171. Zinc6507066
172. Tox21_110193
173. Tox21_500786
174. Bdbm50237593
175. Ccg-40112
176. Nsc757106
177. S1383
178. Stl453211
179. Akos015920078
180. Akos016339636
181. Tox21_110193_1
182. 6-amino-2-imino-4-(piperidin-1-yl)
183. Ac-5271
184. Bcp9000929
185. Ccg-220020
186. Cs-1867
187. Db00350
188. Gs-3605
189. Ks-5164
190. Lp00786
191. Nc00686
192. Sdccgsbi-0050764.p005
193. Idi1_000160
194. Idi1_033855
195. Smp1_000192
196. Ncgc00015673-01
197. Ncgc00015673-02
198. Ncgc00015673-03
199. Ncgc00015673-04
200. Ncgc00015673-05
201. Ncgc00015673-06
202. Ncgc00015673-07
203. Ncgc00015673-09
204. Ncgc00015673-10
205. Ncgc00015673-11
206. Ncgc00015673-13
207. Ncgc00015673-20
208. Ncgc00018278-01
209. Ncgc00018278-02
210. Ncgc00018278-03
211. Ncgc00018278-04
212. Ncgc00024666-01
213. Ncgc00024666-02
214. Ncgc00024666-03
215. Ncgc00024666-04
216. Ncgc00024666-05
217. Ncgc00024666-06
218. Ncgc00024666-07
219. Ncgc00024666-08
220. Ncgc00179672-01
221. Ncgc00261471-01
222. Bd164673
223. Sbi-0050764.p004
224. 2,6-diamino-4-piperidinopyrimidine 1-oxide
225. 5965120sh1
226. Ab00513797
227. Eu-0100786
228. Ft-0620793
229. D00418
230. H10337
231. O10620
232. 6-(1-piperidinyl)-2,4-pyrimidinediamine3-oxide
233. Ab00052047-08
234. Ab00052047_09
235. Ab00052047_10
236. Ab00513797-02
237. 304m915
238. A824098
239. Q424165
240. Q-201408
241. Sr-01000075331-1
242. Sr-01000075331-3
243. Sr-01000075331-5
244. Sr-05000001479-1
245. Sr-05000001479-2
246. 2,4-diamino-6-piperidinopyrimidine 3-oxide.
247. 3-hydroxy-2-imino-6-(1-piperidinyl)-4-pyrimidinamine
248. Brd-k06902185-001-05-2
249. Brd-k06902185-001-10-2
250. Brd-k14888893-001-02-3
251. Minoxidil, British Pharmacopoeia (bp) Reference Standard
252. Z1541638524
253. Minoxidil, European Pharmacopoeia (ep) Reference Standard
254. 2-azanylidene-3-oxidanyl-6-piperidin-1-yl-pyrimidin-4-amine
255. 6-amino-1,2-dihydro-1-hydroxy-2-imino-4-piperidino-pyrimidine
256. Minoxidil, United States Pharmacopeia (usp) Reference Standard
257. Minoxidil For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 209.25 g/mol |
|---|---|
| Molecular Formula | C9H15N5O |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 209.12766012 g/mol |
| Monoisotopic Mass | 209.12766012 g/mol |
| Topological Polar Surface Area | 88.9 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 329 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 16 | |
|---|---|
| Drug Name | Men's rogaine |
| PubMed Health | Minoxidil |
| Drug Classes | Alopecia Agent, Antihypertensive, Peripheral Vasodilator |
| Drug Label | Minoxidil tablets contain minoxidil, an antihypertensive peripheral vasodilator. Minoxidil occurs as a white to off-white, odorless, crystalline solid that is soluble in water to the extent of approximately 2 mg/mL, is readily soluble in propylene gl... |
| Active Ingredient | Minoxidil |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 5% |
| Market Status | Over the Counter |
| Company | Johnson And Johnson |
| 2 of 16 | |
|---|---|
| Drug Name | Minoxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Hi Tech Pharma; Perrigo |
| 3 of 16 | |
|---|---|
| Drug Name | Minoxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Wockhardt; Hi Tech Pharma; Actavis Mid Atlantic; Perrigo |
| 4 of 16 | |
|---|---|
| Drug Name | Minoxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Tablet; Aerosol, foam |
| Route | Oral; Topical |
| Strength | 2.5mg; 5%; 10mg |
| Market Status | Over the Counter; Prescription |
| Company | Par Pharm; Watson Labs; Mutual Pharm; Perrigo Israel |
| 5 of 16 | |
|---|---|
| Drug Name | Minoxidil extra strength |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 5% |
| Market Status | Over the Counter |
| Company | Wockhardt; Avacor Prods; Actavis Mid Atlantic; Perrigo; Perrigo New York |
| 6 of 16 | |
|---|---|
| Drug Name | Rogaine |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Johnson And Johnson |
| 7 of 16 | |
|---|---|
| Drug Name | Rogaine extra strength |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 5% |
| Market Status | Over the Counter |
| Company | Johnson And Johnson |
| 8 of 16 | |
|---|---|
| Drug Name | Theroxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 5%; 2% |
| Market Status | Over the Counter |
| Company | Ei |
| 9 of 16 | |
|---|---|
| Drug Name | Minoxidil extra strength |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 5% |
| Market Status | Over the Counter |
| Company | Wockhardt; Avacor Prods; Actavis Mid Atlantic; Perrigo; Perrigo New York |
| 10 of 16 | |
|---|---|
| Drug Name | Rogaine |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Johnson And Johnson |
| 11 of 16 | |
|---|---|
| Drug Name | Rogaine extra strength |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 5% |
| Market Status | Over the Counter |
| Company | Johnson And Johnson |
| 12 of 16 | |
|---|---|
| Drug Name | Theroxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 5%; 2% |
| Market Status | Over the Counter |
| Company | Ei |
| 13 of 16 | |
|---|---|
| Drug Name | Men's rogaine |
| PubMed Health | Minoxidil |
| Drug Classes | Alopecia Agent, Antihypertensive, Peripheral Vasodilator |
| Drug Label | Minoxidil tablets contain minoxidil, an antihypertensive peripheral vasodilator. Minoxidil occurs as a white to off-white, odorless, crystalline solid that is soluble in water to the extent of approximately 2 mg/mL, is readily soluble in propylene gl... |
| Active Ingredient | Minoxidil |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 5% |
| Market Status | Over the Counter |
| Company | Johnson And Johnson |
| 14 of 16 | |
|---|---|
| Drug Name | Minoxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Hi Tech Pharma; Perrigo |
| 15 of 16 | |
|---|---|
| Drug Name | Minoxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 2% |
| Market Status | Over the Counter |
| Company | Wockhardt; Hi Tech Pharma; Actavis Mid Atlantic; Perrigo |
| 16 of 16 | |
|---|---|
| Drug Name | Minoxidil |
| Active Ingredient | Minoxidil |
| Dosage Form | Tablet; Aerosol, foam |
| Route | Oral; Topical |
| Strength | 2.5mg; 5%; 10mg |
| Market Status | Over the Counter; Prescription |
| Company | Par Pharm; Watson Labs; Mutual Pharm; Perrigo Israel |
Antihypertensive Agents; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Minoxidil is indicated for treatment of hypertension. Because of its serious side effects, minoxidil is not considered to be a primary agent in the treatment of essential hypertension. It is recommended for use only in patients with symptomatic or organ-damaging hypertension not responsive to other treatment. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2077
Minoxidil is used topically to stimulate regrowth of hair in balding areas of individuals with androgenetic alopecia (male pattern alopecia, hereditary alopecia, common male baldness). The drug is effective in promoting hair regrowth on the vertex (crown) of the scalp but appears to have little or no effect on temporal recession; the efficacy of topical minoxidil therapy for frontal alopecia has not been evaluated objectively to date. There is evidence to suggest that individuals most likely to respond to topical minoxidil therapy are those younger than 40 years of age, those in whom treatment is initiated relatively early (less than 10 years' duration of hair loss), those with a small diameter of baldness (less than 10 cm), and those who have a large number of terminal or indeterminate (intermediate) hairs before initiation of treatment. At least 4 mo of continuous therapy with minoxidil topical solution usually is required for evidence of response; however, treatment for up to a year may be warranted before deciding that the alopecia is unresponsive. While the ultimate benefit of topical minoxidil therapy depends on the subjective perceptions of the treated individual, cosmetically acceptable hair regrowth as determined by study investigators has been reported in approximately one third of individuals after 6-12 mo of twice daily therapy in most clinical studies, and complete coverage of the balding areas of the scalp occurs rarely. Current evidence suggests that such therapy must be continued indefinitely for maintenance of hair growth.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3481-2
Topically applied minoxidil has been used as a 1, 3, or 5% solution, ointment, or cream to promote hair regrowth in males and females with alopecia areata, including those with the most severe forms, alopecia totalis (complete loss of scalp hair) or alopecia universalis (complete loss of body hair). Overnight petrolatum occlusion of the treated area, which has been reported to enhance efficacy, has been used in some studies. A cosmetically acceptable response to topical minoxidil therapy appears most likely to occur in patients with patchy alopecia areata; patients with total loss of scalp or body hair at baseline (eg; those with alopecia totalis or universalis) usually have the poorest and most delayed response. Efficacy of any therapy in patients with alopecia areata is difficult to evaluate because of the spontaneous hair regrowth and hair loss characteristic of the disease. As in androgenetic alopecia, some patients with alopecia areata receiving treatment with the vehicle alone in controlled studies have had regrowth of terminal hair, and hair loss has resumed in other patients during continued topical minoxidil therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3482-3
For more Therapeutic Uses (Complete) data for MINOXIDIL (6 total), please visit the HSDB record page.
It is particularly important that the risks versus benefits of topical minoxidil therapy be assessed carefully in individuals older than 50 yr of age; those with cardiac, renal, or hepatic disease or scalp abnormalities; and those receiving potentially interacting drugs concomitantly (eg; hypotensive agents such as guanethidine); such individuals should be closely monitored if a decision is made to initiate therapy. In addition, individuals with underlying cardiac disease, including coronary artery disease or congestive heart failure, should be informed that adverse systemic effects of topical minoxidil therapy may be particularly serious should they occur.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3484
Individuals receiving topical minoxidil therapy and their clinician should be aware of the manifestations of common and otherwise potentially serious adverse effects associated with systemic minoxidil therapy, particularly sodium and water retention, weight gain, local or generalized edema, pericardial effusion, pericarditis, tamponade, tachycardia, and increased frequency or development of angina. ... While such effects generally appear to be unlikely during topical minoxidil therapy, certain individuals may be at increased risk of their development because of underlying disease, sensitivity to the drug, or achievement of higher than usual systemic concentrations (eg; secondary to misuse or enhanced percutaneous penetration of topical drug). Individuals receiving topical minoxidil therapy should be advised to watch for and report the occurrence of increased heart rate, weight gain, difficulty in breathing (especially when lying down), worsening or development of angina pectoris, edema (swelling) of the face, hands, ankles, or abdomen, or other systemic effects and should be monitored 1 mo after initiating therapy and at least every 6 mo thereafter for the possible development of such effects. If systemic effects occur, topical minoxidil therapy should be discontinued.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3484
As with other topically applied drugs, inflammation or disease processes associated with decreased integrity of the epidermal barrier (eg; excoriations of the scalp, severe sunburn, scalp psoriasis) may increase percutaneous absorption of minoxidil and potentially increase the likelihood of systemic adverse effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3484
In evaluating females in whom androgenetic alopecia is suspected, the possibility of an underlying endocrine abnormality such as Cushing's disease, polycystic ovary (Stein-Leventhal) syndrome, hypothyroidism, or an androgen secreting tumor should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3484
For more Drug Warnings (Complete) data for MINOXIDIL (14 total), please visit the HSDB record page.
For the treatment of severe hypertension and in the topical treatment (regrowth) of androgenic alopecia in males and females and stabilisation of hair loss in patients with androgenic alopecia.
FDA Label
Minoxidil is an orally effective direct acting peripheral vasodilator that reduces elevated systolic and diastolic blood pressure by decreasing peripheral vascular resistance. Minoxidil is also used topically to treat androgenetic alopecia. Microcirculatory blood flow in animals is enhanced or maintained in all systemic vascular beds. In man, forearm and renal vascular resistance decline; forearm blood flow increases while renal blood flow and glomerular filtration rate are preserved. The predominant site of minoxidil action is arterial. Venodilation does not occur with minoxidil; thus, postural hypotension is unusual with its administration. The antihypertensive activity of minoxidil is due to its sulphate metabolite, minoxidil sulfate.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
D11AX01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C02 - Antihypertensives
C02D - Arteriolar smooth muscle, agents acting on
C02DC - Pyrimidine derivatives
C02DC01 - Minoxidil
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX01 - Minoxidil
Absorption
Minoxidil is at least 90% absorbed from the GI tract in experimental animals and man.
Minoxidil is almost completely absorbed (95%) from the GI tract. Peak plasma levels are reached in 1 hr. Protein binding does not occur. Minoxidil is widely distributed in the tissues, with an apparent volume of distribution of about 2.8-3.3 l/kg. Placental passage and distribution into breast milk have not been established. The elimination half-life of minoxidil is2.77-4.2 hr. About 90% of an oral dose of minoxidil is metabolized in the liver. The drug and its metabolites are largely excreted in the urine. Renal clearance is 73.9 ml/min.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 314
Percutaneous absorption of minoxidil appears to be minimal following topical application of minoxidil solution to intact scalp. However, systemic absorption of topically applied minoxidil is variable and depends on several factors, including the vehicle used in the formulation, the area of application, condition of the skin (eg; being increased with local abrasion or inflammation), and interindividual variation in the extent of percutaneous absorption. Percutaneous absorption of the drug does not appear to be altered by use of a hot-air hair dryer. Although limited in vitro evidence suggests comparable release of minoxidil from solutions with different proportions of propylene glycol, alcohol, and water, release of the drug from a cream base differs substantially from that from solution formulations, and water-in-oil creams appear to differ markedly from oil-in-water creams or ointments in terms of the rate and concentration dependency of drug permeation through human skin. Absorption of minoxidil from extemporaneously prepared solutions, ointments, creams, or other such topical formulations may not be comparable to that from the commercially available topical minoxidil solution.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3486
Percutaneous absorption of minoxidil following topical application of 2% hydroalcoholic, propylene glycol-containing solutions of the drug generally has been reported to average 0.3-4.5% of the applied dose. In a preliminary study in healthy balding men, the systemic bioavailability of minoxidil 2 or 3% topical solution (20 or 30 mg doses, respectively) at steady state relative to that of a 2.5 mg oral tablet averaged 1.4 or 1.2%, respectively. Based on urinary excretion of radiolabeled drug administered to healthy men in another study, percutaneous absorption of minoxidil after application of 1 or 5% minoxidil solutions to the scalp generally averaged 1.6-3.9% of the applied dose, based on urinary recovery of radiolabeled drug. In studies in animals, 5-36% of topically applied doses was absorbed systemically. Serum concentrations achieved after topical application of minoxidil solutions are variable, and clinical studies of topical minoxidil therapy have found no correlation between serum minoxidil concentrations and hair growth. In controlled studies in individuals with androgenetic alopecia or alopecia areata, serum concentrations of minoxidil after topical application of 1, 2, 3, or 5% solution formulations (with or without nightly petrolatum occlusion) generally averaged 2 ng/ml or less. However, about 1% of individuals receiving a 2% topical solution achieved peak serum concentrations of 5 ng/ml or greater, and a few patients achieved concentrations approaching 30 ng/ml. In part, increased percutaneous absorption of the drug in some individuals may have resulted from alterations in the stratum corneum (eg; secondary to irritation and inflammation from shaving of the scalp). In addition, some individuals may have a propensity for enhanced percutaneous absorption of the drug. Data from healthy balding men indicate that peak serum concentrations of unchanged minoxidil after oral doses of 5 mg daily generally are 20-30 times higher than mean serum concentrations achieved after twice daily topical application of approximately 20 mg (1 ml) of minoxidil as a 2% solution.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3486
The distribution of topically applied minoxidil has not been fully determined. Limited evidence suggests that intact stratum corneum serves as a barrier that inhibits substantial diffusion of topically applied minoxidil into systemic circulation, but additional study is needed. Skin biopsy specimens obtained after topical application of a radiolabeled minoxidil 1 or 5% solution to the scalp of healthy balding men indicate an average retention in the skin of 2.6% or less of the applied dose after 24 hr, with twice as much radioactivity in the dermis as in the epidermis. The remainder of the dose remained on the skin or presumably was lost to the environment.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3486
For more Absorption, Distribution and Excretion (Complete) data for MINOXIDIL (8 total), please visit the HSDB record page.
Approximately 90% of the administered drug is metabolized, predominantly by conjugation with glucuronic acid at the N-oxide position in the pyrimidine ring, but also by conversion to more polar products. Known metabolites exert much less pharmacologic effect than minoxidil itself.
Preliminary evidence suggests that the activity of minoxidil sulfotransferase (the enzyme that converts the drug to its sulfate) is higher in the hair follicle than in epidermis or dermis. Minoxidil sulfate, which may be formed preferentially in the hair follicle following topical application of the drug, exhibits more potent vasodilatory activity than the parent drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3485
About 90% of an oral dose of minoxidil is metabolized, primarily by conjugation with glucuronic acid and also by conversion to more polar metabolites. Minoxidil's metabolites are considerably less active than the parent drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1852
The biotransformation of minoxidil (2,4-diamino-6-piperidinopyrimidine 3-oxide) was studied in the rat, dog, and monkey and compared to reported results in the human. Chromatographic profiles of urinary metabolites show that each species excreted substantially the same metabolites but in quite different relative amounts. The monkey and the human exhibited similar metabolite profiles, whereas the dog and rat were quantitatively different from each other and from the monkey and human. The major excretory product for the monkey and human was a glucuronide conjugate of minoxidil. Substantially smaller amounts of unchanged minoxidil, 2,4-diamino-6-(4'-hydroxypiperidino)pyrimidine 3-oxide, and more polar metabolites also were excreted by these two species. The major excretory product in the rat was unchanged minoxidil. Almost as much (combined) of the two acidic metabolites, 2,4-diamino-6-(4'-carboxy-n-butylamino)pyrimidine and its 3-oxide, also were produced. Smaller amounts of the glucuronide of minoxidil, 2,4-diamino-6-(4'-hydroxypiperidino)pyrimidine 3-oxide, its 3'-hydroxy isomer, and 2,4-diamino-6-piperidinopyrimidine also were excreted by the rat. The major metabolite of minoxidil excreted by the dog was the 4'-hydroxy metabolite. Smaller amounts of unchanged minoxidil and polar metabolites and much smaller amounts of the glucuronide of minoxidil, the 3'-hydroxy metabolite, and 2,4-diamino-6-piperidinopyrimidine also were excreted by the dog. Evidence was obtained for a glucuronide conjugate of the 4'-hydroxy metabolite in this species. The major circulatory material in dog plasma was the 4'-hydroxy metabolite, whereas it was the glucuronide of minoxidil in monkey plasma.
PMID:807713 Thomas RC, Harpootlian H; J Pharm Sci 64 (8): 1366-71 (1975)
Minoxidil has known human metabolites that include 2-Pyrimidinamine, 1,6-dihydro-6-imino-4-(1-piperidinyl)-1-(sulfooxy)-.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4.2 hours
In one study in patients with various degrees of renal function (eg; normal to uremic), the mean plasma half-life of minoxidil and its metabolites was 4.2 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1852
Minoxidil is thought to promote the survival of human dermal papillary cells (DPCs) or hair cells by activating both extracellular signal-regulated kinase (ERK) and Akt and by preventing cell death by increasing the ratio of BCl-2/Bax. Minoxidil may stimulate the growth of human hairs by prolonging anagen through these proliferative and anti-apoptotic effects on DPCs. Minoxidil, when used as a vasodilator, acts by opening adenosine triphosphate-sensitive potassium channels in vascular smooth muscle cells. This vasodilation may also improve the viability of hair cells or hair follicles.
The mechanism(s) by which topically applied minoxidil and/or a metabolite of the drug stimulate vertex hair regrowth in androgenetic (male-pattern) alopecia or other forms of alopecia has not been fully elucidated. However, because minoxidil has stimulated hair regrowth in several forms of alopecia, it appears that the drug acts at the level of the hair follicle, possibly involving direct stimulation of hair follicle epithelial growth. ... While increased scalp blood flow resulting from local vasodilation often has been proposed as a principal mechanism of minoxidil's effect on hair growth, this mechanism has not been substantiated consistently and not all vasodilators produce hypertrichosis.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3485
Studies in animal cell cultures indicate that minoxidil directly induces proliferation of hair epithelial cells near the base of the hair follicle and increases incorporation of cysteine and glycine into the follicle; cysteine residues crosslink to form cystine, which provides strength to the hair shaft. The drug also appears to induce hypertrophy of existing small follicles, prolong the anagen phase of the hair follicle, and accelerate the cyclic turnover of vellus hair follicles, enabling these follicles to produce thick, terminal hair; these effects result in a decrease in vellus hair follicles, an increase in terminal hair follicles, and an increase in the diameter of the hair shaft. Biopsy specimens obtained after topical treatment with minoxidil demonstrate enlargement of preexisting hair follicles but no evidence of new hair follicle formation.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3485
In vitro studies demonstrate different effects of minoxidil on epithelial cells and lymphocytes, which may lead to synergistic effects on hair growth in patients with alopecia areata. In cultures of murine epithelial cells, minoxidil increased cell proliferation, prolonged cell passage time (delayed senescence), and altered cell morphology; the latter 2 effects also have been observed in cultures of human keratinocytes. Human lymphocytes exposed to minoxidil in cell culture demonstrated a modest suppression of mitogen-induced blast formation.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 3486
Minoxidil reduces peripheral vascular resistance and blood pressure as a result of a direct vasodilating effect on vascular smooth muscle; like diazoxide and hydralazine, minoxidil's effect on arterioles is greater than on veins. Minoxidil delays the hydrolysis of cyclic 3',5'-adenosine monophosphate and cyclic guanosine monophosphate by inhibiting the enzyme phosphodiesterase, and relaxation of arterial smooth muscle by the drug may be, at least partly, mediated by cyclic 3',5'-adenosinemonophospate. Animal studies indicate that minoxidil does not have CNS or adrenergic neuronal blocking effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1851

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?