
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
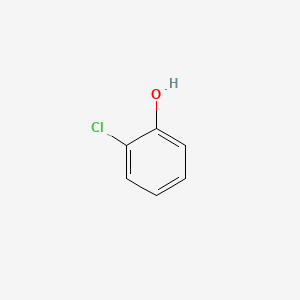

1. 2-monochlorophenol
2. O-chlorophenol
3. O-monochlorophenol
4. Ortho-chlorophenol
5. Ortho-monochlorophenol
1. O-chlorophenol
2. 95-57-8
3. Phenol, 2-chloro-
4. 2-hydroxychlorobenzene
5. O-chlorphenol
6. Chlorophenol
7. 2-chloro-1-hydroxybenzene
8. O-chlorophenic Acid
9. Phenol, O-chloro-
10. 2-chloro-phenol
11. Rcra Waste Number U048
12. Nsc 2870
13. Mfcd00002159
14. K9kav4k6bn
15. 1-chloro-2-hydroxybenzene
16. Chebi:47083
17. Nsc-2870
18. Septi-kleen
19. Dsstox_cid_1544
20. 2-chlorophenol, >=99%
21. Dsstox_rid_76202
22. Dsstox_gsid_21544
23. Pine-o Disinfectant
24. Caswell No. 203
25. O-chlorphenol [german]
26. Ortho-chlorophenol
27. Cas-95-57-8
28. 2ch
29. Ccris 640
30. Hsdb 1415
31. Einecs 202-433-2
32. Unii-k9kav4k6bn
33. Rcra Waste No. U048
34. Epa Pesticide Chemical Code 062204
35. Orthochlorophenol
36. Ai3-09060
37. 2-chloro Phenol
38. 1wbo
39. O-chlorophenol, Solid
40. O-chlorophenol, Liquid
41. Chlorophenol (related)
42. Phenol,2-chloro
43. 2-chlorophenol, 98%
44. Wln: Qr Bg
45. 2-chlorophenol (2-cp)
46. Ec 202-433-2
47. O-chlorophenol [mi]
48. Schembl12279
49. Mls002454425
50. Bidd:er0681
51. 2-chlorophenol [hsdb]
52. Chembl108877
53. Dtxsid5021544
54. Bdbm36301
55. Nsc2870
56. Hms2267f05
57. Zinc402767
58. Amy40778
59. Cs-d1478
60. Tox21_201410
61. Tox21_300340
62. Stl281867
63. Akos000118966
64. Db03110
65. Ncgc00091286-01
66. Ncgc00091286-02
67. Ncgc00091286-03
68. Ncgc00254422-01
69. Ncgc00258961-01
70. 2-chlorophenol 10 Microg/ml In Methanol
71. 25167-80-0
72. Ps-11957
73. Smr000112048
74. 2-chlorophenol 100 Microg/ml In Methanol
75. P-phenylenediamine-o-chlorophenol Copolymer
76. Db-057591
77. 2-chlorophenol 1000 Microg/ml In Methanol
78. Ft-0611987
79. 2-chlorophenol 100 Microg/ml In Isopropanol
80. 2-chlorophenol, Saj First Grade, >=99.0%
81. 2-chlorophenol, Pestanal(r), Analytical Standard
82. Q176486
83. J-509162
84. F0001-2274
85. O-chlorophenol, Solid [un2020] [keep Away From Food]
86. 2-chlorophenol, Certified Reference Material, Tracecert(r)
87. O-chlorophenol, Liquid [un2021] [keep Away From Food]
| Molecular Weight | 128.55 g/mol |
|---|---|
| Molecular Formula | C6H5ClO |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 128.0028925 g/mol |
| Monoisotopic Mass | 128.0028925 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 74.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): ANTISEPTIC
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 106
A number of rabbit studies have shown that metabolism of the monochlorophenols is principally via conjugation. In /one/ study, groups of 6 rabbits were treated by gavage with 171.3 mg/kg of 2-CP or 4-CP emulsified in water as a single dose. For both isomers, the 24-hour urine analysis indicated that between 78.1 and 88.3% of the administered dose was excreted as the glucuronide, and between 12.8 and 20.6% of the administered dose was excreted as the ethereal sulfate. A total of 101.7 and 101.1% of the administered 2-CP or 4-CP doses, respectively, was accounted for as urinary glucuronide and sulfate conjugates.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Chlorophenols PB/99/166639 p.80 (July 1999). Available from, as of October 28, 2008: https://www.atsdr.cdc.gov/toxpro2.html#
... Absorbed from ... gastrointestinal tract & ... parenteral sites of injection. ... Excreted as conjugates of sulfuric & glucuronic acid. ... Urine darkens after standing. /Chlorophenols/
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1615-16
In general, chlorophenols are readily absorbed through the skin. Using the skin of the hairless mouse, aqueous solutions of 2-MCP, 2,4-DCP, and 2,4,6- T3CP readily penetrated the skin, provided that the compound was not ionized. In vitro studies on epidermal membranes from human skin taken at autopsy showed penetration by 2-MCP, 4-MCP, 2,4-DCP, and 2,4,6-T3CP. The lipophilic character of the solutes and their hydrogen-bonding capacity are the 2 main features determining this penetration.
WHO; Environmental Health Criteria Document No. 93: Chlorophenols other than Pentachlorophenol (95-57-8). Available from, as of November 11, 2008: https://www.inchem.org/pages/ehc.html
O-Chlorophenol yields o-chloroanisole in guinea pigs. /In rabbits/ o-chlorophenol yields 3-chlorocatechol. Yields o-chlorophenyl-beta-d-glucuronide & o-chlorophenyl sulfate. O-chlorophenol yields chloroquinol probably in rats.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-36
The urinary and biliary excretion of (14)C-labeled o-chlorophenol were investigated in 12 species of freshwater fish when immersed in sublethal concentrations of the cmpd in the aquarium water for 48 hr. o-Chlorophenol sulfate and o-chlorophenol glucuronide were detected in both the aquarium water and the bile of all the fish species.
PMID:6880238 Layiwola PJ et al; Xenobiotica 13 (2): 107-13 (1983)
... Various chlorophenols are formed as intermediate metabolites during the microbiological degradation of the herbicides 2,4-D & 2,4,5-T and the pesticides Silvex, Ronnel, lindane, and benzene hexachloride. /Chlorophenols/
USEPA; Ambient Water Quality Criteria Doc: Chlorinated Phenols p.A-7 (1980) EPA 440/5-80-032
Mammalian metabolism of chlorobenzene yields 2-chlorophenol as /one of/ the major products.
Smith JRL et al; Xenobiotica 2: 215 (1972) as cited in USEPA; Ambient Water Quality Criteria Doc: Chlorinated Phenols p.C-10 (1980) EPA 440/5-80-032
The major mode of action of chlorophenols appears to be the uncoupling of oxidative phosphorylation. The strength of the uncoupling effect is related to the degree of chlorination: PCP is the strongest inhibitor of oxidative phosphorylation, MCP the weakest. To a lesser extent, inhibition of oxidative phosphorylation is affected by the positions of the chlorine atoms on the molecule. There appears to be a relationship between chlorination and the toxicity of PCP and T4CP, although there is no clear-cut relationship between the degree of chlorination and toxicityin MCP, DCP, and the T3CP series. /chlorophenols/
WHO; Environmental Health Criteria Document No. 93: Chlorophenols other than Pentachlorophenol (95-57-8). Available from, as of November 11, 2008: https://www.inchem.org/pages/ehc.html
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
95
PharmaCompass offers a list of 2-Chlorophenol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right 2-Chlorophenol manufacturer or 2-Chlorophenol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred 2-Chlorophenol manufacturer or 2-Chlorophenol supplier.
PharmaCompass also assists you with knowing the 2-Chlorophenol API Price utilized in the formulation of products. 2-Chlorophenol API Price is not always fixed or binding as the 2-Chlorophenol Price is obtained through a variety of data sources. The 2-Chlorophenol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 2-Chlorophenol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 2-Chlorophenol, including repackagers and relabelers. The FDA regulates 2-Chlorophenol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 2-Chlorophenol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A 2-Chlorophenol supplier is an individual or a company that provides 2-Chlorophenol active pharmaceutical ingredient (API) or 2-Chlorophenol finished formulations upon request. The 2-Chlorophenol suppliers may include 2-Chlorophenol API manufacturers, exporters, distributors and traders.
2-Chlorophenol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 2-Chlorophenol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 2-Chlorophenol GMP manufacturer or 2-Chlorophenol GMP API supplier for your needs.
A 2-Chlorophenol CoA (Certificate of Analysis) is a formal document that attests to 2-Chlorophenol's compliance with 2-Chlorophenol specifications and serves as a tool for batch-level quality control.
2-Chlorophenol CoA mostly includes findings from lab analyses of a specific batch. For each 2-Chlorophenol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
2-Chlorophenol may be tested according to a variety of international standards, such as European Pharmacopoeia (2-Chlorophenol EP), 2-Chlorophenol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (2-Chlorophenol USP).

