
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
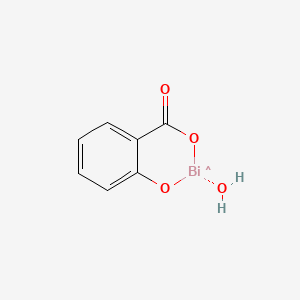

1. Helidac
2. Kaopectate
3. Pepto-bismol
4. Subsalicylate Bismuth
5. Trigastronol
1. 14882-18-9
2. Bismuth Oxysalicylate
3. Bismuth Oxide Salicylate
4. Wismutsubsalicylat
5. Basic Bismuth Salicylate
6. Bismuthi Subsalicylas
7. 4h-1,3,2-benzodioxabismin-4-one, 2-hydroxy-
8. Bismutum Subsalicylicum
9. Bismuth(iii) Subsalicylate
10. Pink Bismuth
11. Bismuth, (2-hydroxybenzoato-o1,o2)oxo-
12. Stabisol
13. Vismut
14. Spiromak Forte
15. Ncgc00166299-01
16. Trigastronol
17. 2-hydroxy-benzo[1,3,2]dioxabismin-4-one
18. Bismuth Oxysalicylate;bismuth(iii) Salicylate Basic
19. 2-hydroxy-2h,4h-benzo[d]1,3-dioxa-2-bismacyclohexan-4-one
20. Bismuth Salicylate, Basic
21. Wismutsalicylat, Basisches
22. Bismuth, Oxo(salicylato)-
23. Bismogenol Tosse Inj
24. Ccris 4751
25. Hsdb 332
26. Salicylic Acid Basic Bismuth Salt
27. Salicylic Acid, Bismuth Basic Salt
28. Einecs 238-953-1
29. Unii-62tey51rr1
30. Bismuth, (2-hydroxybenzoato-o(1),o(2))oxo-
31. Wismutoxidsalicylat
32. 2-hydroxybenzoic Acid Bismuth (3+) Salt, Basic
33. Bismuth-subsalicylate
34. Mfcd00085368
35. Pepto-bismol (tn)
36. Bismuth Subsalicylate [usan:usp:jan]
37. Dsstox_cid_4622
38. 2-hydroxy-4h-1,3,2-benzodioxabismin-4-one
39. Ec 238-953-1
40. Dsstox_rid_77471
41. Dsstox_gsid_24622
42. Mls000069654
43. Dtxsid6024622
44. Bismuth Subsalicylate (jan/usp)
45. Chebi:261649
46. Hms2093h13
47. Pharmakon1600-01505412
48. Hy-b0550
49. Tox21_112398
50. Nsc759117
51. Akos015888405
52. Ccg-213424
53. Db01294
54. Nsc 759117
55. Ncgc00166299-03
56. 2-hydroxy-1,3,2-benzodioxabismin-4-one
57. As-65783
58. Smr000059234
59. Sbi-0206855.p001
60. Cas-14882-18-9
61. C07870
62. D00728
63. Ab00489978_02
64. 2-hydroxy-4h-benzo[d][1,3,2]dioxabismin-4-one
65. Sr-05000001933
66. J-008518
67. Sr-05000001933-1
68. Bismuth(iii) Subsalicylate, 99.9% Trace Metals Basis
69. Bismuth(iii) Salicylate Basic, Purum, >=97.0% (kt)
70. Bismuth Oxysalicylate, Bismuth Subsalicylate, Bismuth(iii) Salicylate Basic
71. Bismuth Subsalicylate, United States Pharmacopeia (usp) Reference Standard
72. 87-27-4
| Molecular Weight | 363.10 g/mol |
|---|---|
| Molecular Formula | C7H6BiO4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 363.00701 g/mol |
| Monoisotopic Mass | 363.00701 g/mol |
| Topological Polar Surface Area | 36.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 173 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
USED MEDICINALLY AS INTESTINAL ABSORBENT.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-93
MEDICATION (VET): ANTIDIARRHEAL. WEAK INTESTINAL ANTISEPTIC DUE TO LIBERATION OF SALICYLIC ACID. USUALLY COMBINED WITH CARBONATES TO MINIMIZE IRRITANT EFFECTS OF FREE ACID WHILE UTILIZING PROTECTIVE EFFECT OF BISMUTH.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 48
THE CMPD IS SOMETIMES USED ORALLY TO ALLAY DIARRHEA OR TO SOOTHE GASTRITIS OR PEPTIC ULCER. ... BEFORE THE ADVENT OF PENICILLIN, BISMUTH SUBSALICYLATE WAS MUCH USED IN THE TREATMENT OF SYPHILIS...
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 2. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 542
/SRP: FORMER/ TREATMENT OF VINCENT'S ANGINA, SYPHILIS
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 8:28
For more Therapeutic Uses (Complete) data for BISMUTH SUBSALICYLATE (7 total), please visit the HSDB record page.
EVEN WHEN ITS USE WAS EXTENSIVE, THE GRADUAL IM INJECTION USED AGAINST SYPHILIS RARELY LED TO SERIOUS POISONING. IT WAS CUSTOMARY TO STOP TREATMENT IF GINGIVITIS, ALBUMINURIA, CUTANEOUS ERUPTIONS, OR MARKED DIARRHEA APPEARED.
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 2. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 543
BIOAVAILABILITY OF DOXYCYCLINE WAS SIGNIFICANTLY REDUCED BY 37% & 51%, RESPECTIVELY, WHEN SUBSALICYLATE BISMUTH WAS GIVEN SIMULTANEOUSLY & AS A MULTIPLE-DOSE REGIMEN BEFORE DOXYCYCLINE. SUBSALICYLATE BISMUTH SHOULD NOT BE TAKEN WHEN DOXYCYCLINE IS USED FOR THERAPEUTIC PURPOSES. AUTHORS SUGGEST TRAVELERS SHOULD NOT TAKE THE AGENTS TOGETHER IN AN EFFORT TO PREVENT DIARRHEA.
PMID:7040708 ERICSSON CD ET AL; JAMA 247 (16): 2266 (1982)
The excretion of large amounts of bismuth obtained from bismuth subsalicylate into breast milk is not expected because of the poor absorption of bismuth into the systemic circulation. Salicylates, however, are excreted in milk and are eliminated more slowly from milk than from plasma with milk:plasma ratios, rising from 0.03-0.08 at 3 hours to 0.34 at 12 hours. Due to the potential for adverse effects in the nursing infant, the American Academy of Pediatrics recommends that salicylates should be used cautiously during breast feeding. A recent review also states that bismuth subsalicylate should be avoided during lactation because of systemic salicylate absorption.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 96
Although the risk for toxicity may be small, significant fetal adverse effects have resulted from chronic exposure to salicylates Because of this, the use of bismuth subsalicyate during gestation should be restricted to the first half of pregnancy and then only in amounts that do not exceed the recommended doses.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 96
3(?). 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-93
Bismuth subsalicylate is indicated to temporarily relieve diarrhea, travelers' diarrhea, and upset stomach due to overindulgence in food and drink, including heartburn, indigestion, nausea, gas, belching, and fullness. Bismuth subsalicylate is a component of HELIDAC Therapy (bismuth subsalicylate, [metronidazole], and [tetracycline]), which is a treatment regimen indicated for the eradication of _H. pylori_ for treatment of patients with _H. pylori_ infection and duodenal ulcer disease.
Bismuth subsalicylate is an antacid and antimicrobial, gastroprotective, anti-secretory, and anti-inflammatory actions. It works to reduce the severity and incidence of flatulence and diarrhea, and consequently relieving gastrointestinal discomfort. In one study, bismuth subsalicylate was prevented traveler's diarrhea with a protection rate >60%. Organobismuth compounds, formed by the breakdown of bismuth subsalicylate in the gastrointestinal tract, inhibit the growth of _Helicobacter pylori_ and other bacteria implicated in gastrointestinal disorders, and some fungi. In one study, bismuth subsalicylate was shown to eradicate up to 90% of _H. pylori_ infection when used as part of a quadruple therapy regimen containing a proton pump inhibitor, tetracycline, and metronidazole. Bismuth subsalicylate exhibited antimicrobial activity against _Clostridium difficile_, enterotoxigenic _Escherichia coli_ O157:H7, _norovirus_, and other common enteric pathogens such as _Salmonella_ and _Shigella_.
Antidiarrheals
Miscellaneous agents found useful in the symptomatic treatment of diarrhea. They have no effect on the agent(s) that cause diarrhea, but merely alleviate the condition. (See all compounds classified as Antidiarrheals.)
Absorption
Following oral administration, bismuth subsalicylate hydrolyzes into bismuth and salicylic acid in the stomach. Salicylic acid is almost completely absorbed in the small intestine and reaches plasma peak levels one to two hours after dosing. In one study involving healthy male subjects, oral administration of 60 mL Pepto-Bismol, a common over-the-counter product of bismuth subsalicylate, equivalent to 1050 mg of bismuth subsalicylate, resulted in the peak plasma concentration of salicylate of 40.1 g/mL, with a time to peak concentration (Tmax) of 1.8 hours. Less than 1% of bismuth from bismuth subsalicylate is absorbed from the gastrointestinal tract into the systemic circulation. In one study, oral administration of 787 mg bismuth subsalicylate in the chewable tablet form for two weeks resulted in the mean trough blood bismuth concentration was 5.1 3.1 ng/mL. In another study, the mean trough blood bismuth concentration ranged from five to 32 ng/mL following oral administration of 525 mg bismuth subsalicylate in the liquid suspension form.
Route of Elimination
Following oral administration, salicylate dissociated from bismuth subsalicylate is excreted in the urine. Bismuth is primarily eliminated via urinary and biliary routes.
Volume of Distribution
There is no information available.
Clearance
The renal clearance of bismuth is 50 18 mL/min.
THE AUTOPSY DISTRIBUTION OF BISMUTH IN 22 PATIENTS WHO RECEIVED THERAPEUTIC IM INJECTIONS (MAINLY BISMUTH SALICYLATE) WAS AS FOLLOWS (MEDIAN VALUES, MG/KG, WET WEIGHT): KIDNEY 33.3; LIVER 6.8; SPLEEN 1.6; COLON 1.2; LUNG 0.9; BRAIN 0.6 & BLOOD 0.5.
Friberg, L., Nordberg, G.F., Kessler, E. and Vouk, V.B. (eds). Handbook of the Toxicology of Metals. 2nd ed. Vols I, II.: Amsterdam: Elsevier Science Publishers B.V., 1986., p. 121
In the gastrointestinal tract, bismuth subsalicylate is converted to salicylic acid and insoluble bismuth salts. The salicylate portion of bismuth subsalicylate is extensively absorbed (greater than 90%) and excreted in urine.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1951
Bismuth subsalicylate (bismuth salicylate) is hydrolyzed in the gastrointestinal tract to bismuth salts and sodium salicylate. Two tablets or 30 ml suspension of the compound yields 204 mg and 258 mg, respectively, of salicylate. Inorganic bismuth salts, in contrast to organic complexes of bismuth, are relatively water-insoluble and poorly absorbed systemically, but significant absorption of salicylate does occur. A brief 1992 study found minimal absorption of bismuth (exact serum concentrations not specified) from bismuth subsalicylate in 12 healthy subjects, as opposed to a peak serum level of 0.050 ug/ml after a dose of 216 mg of colloidal bismuth subcitrate in a single patient. Some bismuth absorption was documented across the normal gastric mucosa, but the primary absorption occurred from the duodenum.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 95
Bismuth subsalicylate undergo hydrolysis at pH levels lesser than three. It is largely hydrolyzed in the stomach to bismuth oxychloride and salicylic acid. In the small intestine, unchanged bismuth subsalicylate reacts with other anions such as bicarbonate and phosphate to form insoluble bismuth salts. In the colon, unchanged bismuth subsalicylate and other bismuth salts react with hydrogen sulfide produced by anaerobic bacteria to form bismuth sulfide, a highly insoluble black salt responsible for the darkening of the stools.
The terminal half-life of salicylic acid following a single oral dose of 525 mg bismuth subsalicylate is ranges from two to five hours. Bismuth has an intermediate half-life of 5 to 11 days and a terminal half-life of 21 to 72 days.
The exact mechanism of bismuth subsalicylate is not fully understood. Bismuth subsalicylate is an insoluble complex that constitutes salicylic acid and trivalent bismuth. Once orally administered, bismuth subsalicylate hydrolyzes in the stomach into bismuth oxychloride, which is minimally absorbed into the bloodstream, and salicylic acid, which is almost completely absorbed. Bismuth interacts with other anions and compounds, such as hydrochloric acid, bicarbonate, phosphate, and hydrogen sulfide, in the gastrointestinal tract to form bismuth salts such as bismuth oxychloride, bismuth subcarbonate, bismuth phosphate, and bismuth sulfide. Bismuth salts possess bactericidal and antimicrobial activity, mainly by preventing bacteria from binding and growing on the mucosal cells of the stomach. It has no effects on normal gut flora. By preventing bacteria from binding to mucosal cells, bismuth subsalicylate prevents intestinal secretion and fluid loss, promotes fluid and electrolyte reabsorption, reduces gastrointestinal inflammation, and promotes the healing of pre-existing ulcer in the stomach. Salicylic acid from dissociated bismuth subsalicylate adds to the anti-inflammatory actions of bismuth salts by inhibiting the cyclooxygenase enzyme and limiting the formation of prostaglandin, a pro-inflammatory mediator. Bismuth subsalicylate exhibits cytoprotective and demulcent activity, which makes it an effective drug in peptic ulcer disease. It blocks the adhesion of H. pylori to the gastric epithelial cells and blocks the bacteria's enzyme activities, including phospholipase, protease, and urease.
IN THE Y-1 ADRENAL CELL TISSUE CULTURE SYSTEM, A PREPN CONTAINING BISMUTH SUBSALICYLATE REDUCED THE ACTIVITY OF CRUDE TOXIN FROM VIBRIO CHOLERAE BY 104-FOLD AS COMPARED WITH THE ACTIVITY OF CONTROLS. SIMILAR RESULTS WERE OBTAINED USING THE ADULT RABBIT LIGATED INTESTINAL LOOP MODEL. THE PREPN FAILED TO AFFECT CRUDE ESCHERICHIA COLI OR CHOLERA TOXIN ACTIVITY ONCE THESE TOXINS HAD BECOME BOUND TO INTESTINAL MUCOSA.
PMID:335003 ERICSSON CD ET AL; J INFECT DIS 136 (5): 693 (1977)
ATTAPULGITE & PEPTO-BISMOL WERE EFFECTIVE IN REDUCING FLUID ACCUMULATION IN LIGATED SEGMENTS OF PIG INTESTINE INFECTED WITH ENTEROPATHOGENIC ESCHERICHIA COLI. FOR PEPTO-BISMOL THIS EFFECT WAS ASSOCIATED WITH AN ANTIBACTERIAL AS WELL AS AN ANTITOXIC EFFECT, PROBABLY DUE TO ITS ABSORBENT PROPERTIES. AN ASPIRIN-LIKE EFFECT IN THE GUT DUE TO THE ACTIVE INGREDIENT BISMUTH SUBSALICYLATE MAY HAVE CONTRIBUTED TO THE EFFECTIVENESS OF PEPTO-BISMOL.
PMID:356940 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1277637 GYLES CL, ZIGLER M; CAN J COMP MED 42 (3): 260 (1978)

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25360
Submission : 2011-10-04
Status : Active
Type : II

NDC Package Code : 57465-200
Start Marketing Date : 1986-01-02
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27871
Submission : 2013-12-19
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9660
Submission : 1992-04-20
Status : Active
Type : II

NDC Package Code : 47167-4444
Start Marketing Date : 1992-01-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (20kg/20kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16327
Submission : 2002-12-23
Status : Active
Type : II

NDC Package Code : 47167-4444
Start Marketing Date : 1992-01-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (20kg/20kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36384
Submission : 2021-12-15
Status : Active
Type : II

NDC Package Code : 47167-4444
Start Marketing Date : 1992-01-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (20kg/20kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11889
Submission : 1996-03-11
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 572
Submission : 1963-05-29
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9660
Submission : 1992-04-20
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36384
Submission : 2021-12-15
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16327
Submission : 2002-12-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27871
Submission : 2013-12-19
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25360
Submission : 2011-10-04
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11889
Submission : 1996-03-11
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 572
Submission : 1963-05-29
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 47167-5555
Start Marketing Date : 2002-01-04
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 47167-4444
Start Marketing Date : 1992-01-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (20kg/20kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 47167-8888
Start Marketing Date : 2021-11-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (500kg/500kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 57465-200
Start Marketing Date : 1986-01-02
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 62991-1571
Start Marketing Date : 2017-03-06
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 54474-0001
Start Marketing Date : 2012-12-04
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 51927-0283
Start Marketing Date : 2013-07-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

NDC Package Code : 58624-0711
Start Marketing Date : 2024-08-22
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
BISMUTH SUBSALICYLATE; METRONIDAZOLE; TETRACYCLINE HYDROCHLORIDE
Brand Name : BISMUTH SUBSALICYLATE, METRONIDAZOLE AND TETRACYCLINE HYDROCHLORIDE
Dosage Form : TABLET, CHEWABLE, TABLET, CAPSULE;ORAL
Dosage Strength : 262.4MG, N/A, N/A;N/A, 250MG, N/A;N/A, N/A, 500MG
Packaging :
Approval Date : 2018-11-30
Application Number : 202584
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country :
Bismuth Subsalicylate; Ranitidine Hydrochloride
Brand Name : Peptimax
Dosage Form : Tablet
Dosage Strength : 131.5MG; 12.5MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : BISMUTH SUBSALICYLATE CAPLETS
Dosage Form : TABLET
Dosage Strength : 262MG
Packaging : 24
Approval Date :
Application Number : 2326582
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : BISMUTH SUBSALICYLATE SUSPENSION
Dosage Form : SUSPENSION
Dosage Strength : 17.5MG/ML
Packaging : 230ML/480ML
Approval Date :
Application Number : 2313162
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : EXTRA STRENGTH BISMUTH
Dosage Form : SUSPENSION
Dosage Strength : 35.2MG/ML
Packaging : 350ML
Approval Date :
Application Number : 2475855
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : REGULAR STRENGTH BISMUTH
Dosage Form : SUSPENSION
Dosage Strength : 17.5MG/ML
Packaging : 115/230/236/350/480ML
Approval Date :
Application Number : 2468646
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : BISMUTH CAPLETS
Dosage Form : TABLET
Dosage Strength : 262MG
Packaging : 24
Approval Date :
Application Number : 2457121
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO BISMOL CAPLET - 262MG/CAPLET
Dosage Form : TABLET
Dosage Strength : 262MG
Packaging : 24 CAPLETS
Approval Date :
Application Number : 2177994
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO-BISMOL EXTRA
Dosage Form : SUSPENSION
Dosage Strength : 35.2MG/ML
Packaging : 350ML
Approval Date :
Application Number : 2229668
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO-BISMOL EXTRA CAPLET
Dosage Form : TABLET
Dosage Strength : 524MG/TAB
Packaging :
Approval Date :
Application Number : 2530759
Regulatory Info : OTC
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
BISMUTH SUBSALICYLATE; METRONIDAZOLE; TETRACYCLINE HYDROCHLORIDE
Brand Name : BISMUTH SUBSALICYLATE, METRONIDAZOLE AND TETRACYCLINE HYDROCHLORIDE
Dosage Form : TABLET, CHEWABLE, TABLET, CAPSULE;ORAL
Dosage Strength : 262.4MG, N/A, N/A;N/A, 250MG, N/A;N/A, N/A, 500MG
Approval Date : 2018-11-30
Application Number : 202584
RX/OTC/DISCN : RX
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
BISMUTH SUBSALICYLATE; METRONIDAZOLE; TETRACYCLINE HYDROCHLORIDE
Brand Name : HELIDAC
Dosage Form : TABLET, CHEWABLE, TABLET, CAPSULE;ORAL
Dosage Strength : 262.4MG,N/A,N/A;N/A,250MG,N/A;N/A,N/A,500MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1996-08-15
Application Number : 50719
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : BISMUTH SUBSALICYLATE CAPLETS
Dosage Form : TABLET
Dosage Strength : 262MG
Packaging : 24
Approval Date :
Application Number : 2326582
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : BISMUTH SUBSALICYLATE SUSPENSION
Dosage Form : SUSPENSION
Dosage Strength : 17.5MG/ML
Packaging : 230ML/480ML
Approval Date :
Application Number : 2313162
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : EXTRA STRENGTH BISMUTH SUBSALICYLATE SUSPENSION
Dosage Form : SUSPENSION
Dosage Strength : 35MG/ML
Packaging : 236/350/473ML
Approval Date :
Application Number : 2328453
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : STOMACH RELIEF CHEWABLE TABLETS
Dosage Form : TABLET (CHEWABLE)
Dosage Strength : 262MG
Packaging : 24/48
Approval Date :
Application Number : 2389215
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : REGULAR STRENGTH BISMUTH
Dosage Form : SUSPENSION
Dosage Strength : 17.5MG/ML
Packaging : 115/230/236/350/480ML
Approval Date :
Application Number : 2468646
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : BISMUTH CAPLETS
Dosage Form : TABLET
Dosage Strength : 262MG
Packaging : 24
Approval Date :
Application Number : 2457121
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO BISMOL CAPLET - 262MG/CAPLET
Dosage Form : TABLET
Dosage Strength : 262MG
Packaging : 24 CAPLETS
Approval Date :
Application Number : 2177994
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO-BISMOL
Dosage Form : SUSPENSION
Dosage Strength : 17.6MG/ML
Packaging : 115/230/350/480ML
Approval Date :
Application Number : 2097079
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO-BISMOL CHEWABLES
Dosage Form : TABLET (CHEWABLE)
Dosage Strength : 262MG
Packaging : 12/24/48
Approval Date :
Application Number : 2237315
Regulatory Info : OTC
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : OTC
Registration Country : Canada
Brand Name : PEPTO BISMOL LIQUICAPS
Dosage Form : CAPSULE
Dosage Strength : 262MG
Packaging :
Approval Date :
Application Number : 2499991
Regulatory Info : OTC
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?