
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
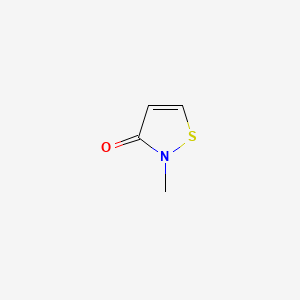

1. 2-methyl-4-isothiazolin-3-one Hydrochloride
2. 243-k-cg
3. Methyl-isothiazolinone
4. Methylisothiazolidinone
5. Methylisothiazolinone
6. Methylisothiazolone
7. N-methylisothiazolone
1. 2682-20-4
2. Methylisothiazolinone
3. 2-methylisothiazol-3(2h)-one
4. 2-methyl-3(2h)-isothiazolone
5. 2-methyl-4-isothiazoline-3-one
6. 3(2h)-isothiazolone, 2-methyl-
7. 2-methyl-1,2-thiazol-3-one
8. N-methyl-3-oxodihydroisothiazole
9. 2-methyl-3-isothiazolone
10. 2-methyl-1,2-thiazol-3(2h)-one
11. N-methyl-3-oxodihydro Isothiazole
12. Mit
13. 229d0e1qfa
14. Chebi:53620
15. N-methylisothiazolin-3-one
16. 2682-20-4 (free Base)
17. Neolone
18. Caswell No. 572a
19. 2-methyl-2h-isothiazol-3-one
20. Microcare Mt
21. Kordek Mlx
22. Einecs 220-239-6
23. Mit (biocide)
24. Acticide M 10
25. Acticide M 20
26. Bestcide 600
27. Kordek 50
28. Kordek 50c
29. Kordek 573f
30. Kathon Cg 243
31. Unii-229d0e1qfa
32. Hsdb 8200
33. Mit 950
34. Mt 10
35. 2-methyl-4-isothiazoline-3-ketone
36. Kb 838
37. Mfcd01742315
38. 2-methyl-4-isothiazolin-3-one (mi)
39. 2-methyl-4-isothiazolinone
40. Dsstox_cid_14259
41. Dsstox_gsid_34259
42. Schembl17863
43. Schembl113898
44. Methylisothiazolinone Free Base
45. Chembl1620780
46. Dtxsid2034259
47. 2-methyl 4-isothiazoline 3-one
48. Methylisothiazolinone [ii]
49. Methylisothiazolinone [mi]
50. 2-methyl-3(2h)-isothiazolone #
51. Methylisothiazolinone [inci]
52. Zinc1849933
53. Methylisothiazolinone [vandf]
54. Tox21_303814
55. Bbl104136
56. Methylisothiazolinone [mart.]
57. Stl557951
58. Akos007930246
59. Am84857
60. Cs-w011236
61. Hy-w010520
62. 2-methyl-4-isothiazolin-3-one, 95%
63. Ncgc00357093-01
64. Cas-2682-20-4
65. Db-005250
66. Ft-0601044
67. F21330
68. 682m204
69. Q423870
70. 2-methyl-4-isothiazolin-3-one, Analytical Standard
71. W-107150
72. 2-methyl-4-isothiazolin-3-one, 50% Aqueous Solution
73. 2-methyl-4-isothiazolin-3-one 100 Microg/ml In Methanol
74. 2-methyl-4-isothiazolin-3-one 1000 Microg/ml In Methanol
75. 2-methyl-4-isothiazolin-3-one 100 Microg/ml In Acetonitrile
| Molecular Weight | 115.16 g/mol |
|---|---|
| Molecular Formula | C4H5NOS |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 115.00918496 g/mol |
| Monoisotopic Mass | 115.00918496 g/mol |
| Topological Polar Surface Area | 45.6 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Disinfectants
Substances used on inanimate objects that destroy harmful microorganisms or inhibit their activity. Disinfectants are classed as complete, destroying SPORES as well as vegetative forms of microorganisms, or incomplete, destroying only vegetative forms of the organisms. They are distinguished from ANTISEPTICS, which are local anti-infective agents used on humans and other animals. (From Hawley's Condensed Chemical Dictionary, 11th ed) (See all compounds classified as Disinfectants.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Neurodegenerative disorders in humans may be triggered or exacerbated by exposure to occupational or environmental agents. ...a brief exposure to methylisothiazolinone, a widely used industrial and household biocide, is highly toxic to cultured neurons but not to glia. /The study/ also show that the toxic actions of this biocide are zinc dependent and require the activation of p44/42 extracellular signal-regulated kinase (ERK) via a 12-lipoxygenase-mediated pathway. The cell death process also involves activation of NADPH oxidase, generation of reactive oxygen species, DNA damage, and overactivation of poly(ADP-ribose) polymerase, all occurring downstream from ERK phosphorylation. The toxic effects of methylisothiazolinone and related biocides on neurons have not been reported previously. Because of their widespread use, the neurotoxic consequences of both acute and chronic human exposure to these toxins need to be evaluated.
PMID:12196562 Du S et al; J Neurosci 22 (17): 7408-16 (2002)
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase (PTK) which acts as an early modulator in the integrin signaling cascade. FAK phosphorylation and its consequent activation regulate several basic biological cellular functions. On the contrary, dysregulation of FAK signaling is implicated in the malignant transformation of cells, as well as in nonmalignant pathological conditions. With respect to cytotoxicity, accumulating data indicate that FAK participates in the mechanism of action of the known cytotoxic reactive oxygen species (ROS). Additionally, evidence was presented that different cytotoxic substances, such as arsenic (As), lead (Pb), acrylamide, methylisothiazolinone (MIT), dichlorovinylcysteine (DCVC) and halothane, acted, at least in part, by downregulating FAK tyrosine phosphorylation, while the bacterial toxins Pasteurella multocida toxin and Escherichia coli cytotoxic necrotizing factor, have been shown to exert cytotoxic effects by inducing FAK tyrosine phosphorylation. The observation that upregulation as well as downregulation of FAK activity both result in cytotoxic effects seems contradictory. Even though a common mode of action, with respect to the dysregulation of FAK signaling, for these cytotoxic substances has not yet been discovered, a cumulative approach could be established by focusing on FAK activation and signaling cascade. According to these data, interfering with FAK signaling might be of a potential use in blocking these cytotoxic effects.
PMID:18215454 Chatzizacharias NA et al; Toxicology 245 (1-2): 1-10 (2008)
Methylisothiazolinone (MIT) is a biocide widely used in industrial and cosmetic products with potential as a neurotoxicant. /it was/ previously reported that short acute exposures to relatively high concentrations of MIT (100 uM) lead to widespread and selective neuronal death in vitro. To evaluate the biological properties of chronic exposures to MIT, freshly dissociated rat cortical neurons were continuously exposed to low concentrations (0.1-3 uM) of the biocide in serum-containing media. Although /this study/ observed minimal effects on cell viability, MIT induced a dramatic inhibition of neurite outgrowth. Immunoblotting and immunoprecipitation experiments revealed that focal adhesion kinase (FAK) phosphorylation was primarily affected by the MIT treatment. The phosphorylation level at tyrosines 576 and 861 of FAK was significantly decreased and likely contributed to the overall reduction of tyrosine phosphorylation of this protein. MIT inhibited Src family kinases (SFKs) in cell-free assays and led to the physical dissociation of FAK from the signaling complexes that it normally forms with c-Src and Fyn in developing neurons. High-density neuronal cultures were then employed to increase cell-to-cell contact. This approach resulted in an overall enhancement of SFKs and FAK phosphorylation and could overcome the deficits induced by MIT. This study suggests that a disruption of FAK-SFK complexes due to SFK inhibition leads to FAK dysfunction, with detrimental effects to immature neurons. Prolonged exposure to low levels of MIT and related compounds may have damaging consequences to the developing nervous system.
PMID:16547166 He K et al; J Pharmacol Exp Ther 317 (3): 1320-9 (2006)
Market Place

ABOUT THIS PAGE
78
PharmaCompass offers a list of 2-Methyl-4-Isothiazolin-3-One API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right 2-Methyl-4-Isothiazolin-3-One manufacturer or 2-Methyl-4-Isothiazolin-3-One supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred 2-Methyl-4-Isothiazolin-3-One manufacturer or 2-Methyl-4-Isothiazolin-3-One supplier.
PharmaCompass also assists you with knowing the 2-Methyl-4-Isothiazolin-3-One API Price utilized in the formulation of products. 2-Methyl-4-Isothiazolin-3-One API Price is not always fixed or binding as the 2-Methyl-4-Isothiazolin-3-One Price is obtained through a variety of data sources. The 2-Methyl-4-Isothiazolin-3-One Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 2-Methyl-4-Isothiazolin-3-One manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 2-Methyl-4-Isothiazolin-3-One, including repackagers and relabelers. The FDA regulates 2-Methyl-4-Isothiazolin-3-One manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 2-Methyl-4-Isothiazolin-3-One API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A 2-Methyl-4-Isothiazolin-3-One supplier is an individual or a company that provides 2-Methyl-4-Isothiazolin-3-One active pharmaceutical ingredient (API) or 2-Methyl-4-Isothiazolin-3-One finished formulations upon request. The 2-Methyl-4-Isothiazolin-3-One suppliers may include 2-Methyl-4-Isothiazolin-3-One API manufacturers, exporters, distributors and traders.
2-Methyl-4-Isothiazolin-3-One Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 2-Methyl-4-Isothiazolin-3-One GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 2-Methyl-4-Isothiazolin-3-One GMP manufacturer or 2-Methyl-4-Isothiazolin-3-One GMP API supplier for your needs.
A 2-Methyl-4-Isothiazolin-3-One CoA (Certificate of Analysis) is a formal document that attests to 2-Methyl-4-Isothiazolin-3-One's compliance with 2-Methyl-4-Isothiazolin-3-One specifications and serves as a tool for batch-level quality control.
2-Methyl-4-Isothiazolin-3-One CoA mostly includes findings from lab analyses of a specific batch. For each 2-Methyl-4-Isothiazolin-3-One CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
2-Methyl-4-Isothiazolin-3-One may be tested according to a variety of international standards, such as European Pharmacopoeia (2-Methyl-4-Isothiazolin-3-One EP), 2-Methyl-4-Isothiazolin-3-One JP (Japanese Pharmacopeia) and the US Pharmacopoeia (2-Methyl-4-Isothiazolin-3-One USP).
