
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
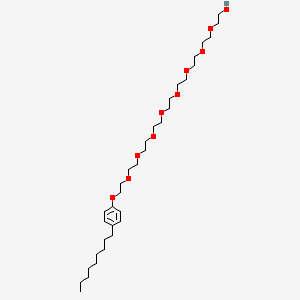

1. Advantage S
2. Advantage-s
3. Delfen Cream
4. Delfen Creams
5. Emulgen 911
6. Emulgin 913
7. Nonoxinol
8. Nonoxinols
9. Nonoxynol
10. Nonoxynol 9
11. Nonoxynol, 1(4)-sulfate, Sodium Salt
12. Nonoxynol, 4-sulfate, Ammonium Salt
13. Nonoxynols
14. Nonylphenoxypolyethoxyethanol
15. Nonylphenoxypolyethoxyethanols
16. Oval, Patentex
17. Patentex Oval
1. Nonoxynol 9
2. 14409-72-4
3. Delfen
4. Nonoxynol, N=9
5. 26571-11-9
6. Tergitol Np-9
7. Peg-9 Nonyl Phenyl Ether
8. Nonoxynol9
9. Tergitol Np9
10. Nonaethylene Glycol Nonylphenyl Ether
11. Nonylphenol Octa(oxyethylene) Ethanol
12. Nonaethylene Glycol Mono(nonylphenyl) Ether
13. Nonoxinol-9
14. 2-[2-[2-[2-[2-[2-[2-[2-[2-(4-nonylphenoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol
15. Nonylphenoxypolyethoxyethanol
16. Polyoxyethylene (9) Nonyl Phenyl Ether
17. 26-(4-nonylphenoxy)-3,6,9,12,15,18,21,24-octaoxahexacosan-1-ol
18. 26-(nonylphenoxy)-3,6,9,12,15,18,21,24-octaoxahexacosan-1-ol
19. Chebi:53775
20. Nsc-758941
21. P-nonylphenyl Polyethylene Glycol Ether
22. N-9
23. Conceptrol
24. Intercept
25. Semicid
26. 3,6,9,12,15,18,21,24-octaoxahexacosan-1-ol, 26-(4-nonylphenoxy)-
27. 2-[2-[2-[2-[2-[2-[2-[2-[2-(4-nonylphenoxy)ethoxy]ethoxy]ethoxy]ethoxy]-ethoxy]ethoxy]ethoxy]ethoxy]ethanol
28. Staycept
29. Emko
30. Today Sponge
31. Nonoxinolum 9
32. Hsdb 8094
33. Gynol Ii
34. C-film
35. Norfox Np-9
36. Tergitol Tp-9
37. Nonoxynol-9.5
38. Einecs 247-816-5
39. Conco Ni-90
40. K-y Plus Nonoxynol-9
41. Spectrum2_001247
42. Spectrum3_001946
43. Alosetronhydrochloride
44. Chembl1410
45. Schembl36844
46. Bspbio_003546
47. Spectrum1505292
48. Spbio_001154
49. Dowfax 9n9, Neutronyx 611
50. Co-630sp
51. Kbio3_002833
52. Dtxsid00858720
53. Hms2093o22
54. Pharmakon1600-01505292
55. Zinc8214629
56. 3,6,9,12,15,18,21,24-octaoxahexacosan-1-ol, 26-(nonylphenoxy)-
57. Bdbm50442874
58. Ccg-39124
59. Nsc758941
60. Akos015910808
61. Db06804
62. Nsc 758941
63. Ncgc00095907-02
64. Ncgc00095907-03
65. N 9
66. Sbi-0206761.p001
67. Polyethylene Glycol 450 Nonyl Phenyl Ether
68. Ab01563056_01
69. Q420039
70. Sr-05000001885
71. J-016227
72. Q-201490
73. Sr-05000001885-1
74. Brd-k88625236-001-01-8
75. 1-(4-nonylphenyl)-1,4,7,10,13,16,19,22,25-nonaoxaheptacosan-27-ol
76. N9
| Molecular Weight | 616.8 g/mol |
|---|---|
| Molecular Formula | C33H60O10 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 35 |
| Exact Mass | 616.41864811 g/mol |
| Monoisotopic Mass | 616.41864811 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 520 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Today |
| PubMed Health | Nonoxynol 9 (Vaginal) |
| Drug Classes | Contraceptive, Local |
| Active Ingredient | Nonoxynol-9 |
| Dosage Form | Sponge |
| Route | Vaginal |
| Strength | 1gm |
| Market Status | Over the Counter |
| Company | Mayer Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Today |
| PubMed Health | Nonoxynol 9 (Vaginal) |
| Drug Classes | Contraceptive, Local |
| Active Ingredient | Nonoxynol-9 |
| Dosage Form | Sponge |
| Route | Vaginal |
| Strength | 1gm |
| Market Status | Over the Counter |
| Company | Mayer Labs |
In January, 2003, FDA proposed new warning statements and other labeling information for these products after results from a major clinical study in Africa and Thailand showed that women using a contraceptive gel product containing N9 were not protected against HIV and other STDs and were, in fact, at higher risk for HIV infection than women using a placebo gel. Because these and other studies have shown that use of products containing N9 cause vaginal and rectal irritation that can heighten the chance of becoming infected with HIV from an infected partner, FDA believes the warning will empower consumers to make better informed decisions about the use of these products, and better protect the public health.
DHHS/FDA; For Consumers: New Warning for Nonoxynol 9 OTC Contraceptive Products re: STDS and HIV/AIDS (June18, 2009). Available from, as of October 16, 2012: https://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124023.htm
The FDA issued a final rule on December 18, 2007 requiring manufacturers of over-the-counter (OTC) stand-alone vaginal contraceptive and spermicidal products containing the chemical ingredient nonoxynol 9 (N9) include a warning stating that the chemical N9 does not provide protection against infection from HIV (the virus that causes AIDS) or other sexually transmitted diseases (STDs). Stand-alone spermicides include gels, foams, films, or inserts containing N9 that are used by themselves for contraception. Consumers can protect themselves from the transmission of STDs and HIV by practicing abstinence, being in a monogamous relationship where neither partner is infected, and using condoms consistently and correctly. FDA is issuing the rule in an effort to correct misconceptions that N9 protects against sexually transmitted diseases, including HIV infection.
DHHS/FDA; For Consumers: New Warning for Nonoxynol 9 OTC Contraceptive Products re: STDS and HIV/AIDS (June18, 2009). Available from, as of October 16, 2012: https://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124023.htm
Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected partner.
DHHS/FDA; For Consumers: New Warning for Nonoxynol 9 OTC Contraceptive Products re: STDS and HIV/AIDS (June18, 2009). Available from, as of October 16, 2012: https://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124023.htm
Do not use if you or your sex partner has HIV/AIDS. If you do not know if you or your sex partner is infected, choose another form of birth control.
DHHS/FDA; For Consumers: New Warning for Nonoxynol 9 OTC Contraceptive Products re: STDS and HIV/AIDS (June18, 2009). Available from, as of October 16, 2012: https://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124023.htm
For more Drug Warnings (Complete) data for Nonoxynol-9 (7 total), please visit the HSDB record page.
Nonoxynol 9 is a surfactant spermicide used for contraception in spermicidal creams, jellies, foams, gel, and lubricants. It is also used in conjuction with other methods of contraception, including condoms, cervical caps and diaphragms.
Surface-Active Agents
Agents that modify interfacial tension of water; usually substances that have one lipophilic and one hydrophilic group in the molecule; includes soaps, detergents, emulsifiers, dispersing and wetting agents, and several groups of antiseptics. (See all compounds classified as Surface-Active Agents.)
Spermatocidal Agents
Chemical substances that are destructive to spermatozoa used as topically administered vaginal contraceptives. (See all compounds classified as Spermatocidal Agents.)
In mice intravenously injected with (14)C-Nonoxynol-9, the highest amounts of radioactivity were reported for the small and large intestines. (14)C radioactivity was excreted mainly in the urine and feces. An HPLC analysis of the urine and bile indicated that no intact Nonoxynol-9 was present in the bile or urine, and that Nonoxynol-9 was metabolized to highly polar species.
Alan AF ed.; Cosmetic Ingredient Review: Amended final report on the safety assessment of Nonoxynol -1, -2, -3, -4, -5, -6, -7 and -8. Int J Toxicol 18 (Suppl 1):11-31 (1999)
In in vitro skin penetration studies using cadaver skin (rinse off and leave-on protocols), the total skin penetration of Nonoxynol-2, -4, and -9 was less than 1 % over a period of 48 hours.
Alan AF ed.; Cosmetic Ingredient Review: Amended final report on the safety assessment of Nonoxynol -1, -2, -3, -4, -5, -6, -7 and -8. Int J Toxicol 18 (Suppl 1):11-31 (1999)
The disposition of nonoxynol-9 labeled with (14)C at the ethylene oxide units was studied following an iv or vaginal administration to female Sprague-Dawley rats. The results from the vaginal administration studies indicate 12.8% absorption of (14)C radioactivity in 6.0 hr and 37.7% in 24.0 hr. Tissue distribution studies showed that the small and large intestines, including their contents, had the highest (14)C activity by either route of administration. Radiomonitored HPLC of bile collected at 6.0 hr and urine at 6.0, 24.0, and 48.0 hr following an iv injection of (14)C nonoxynol-9 showed that the compound was completely metabolized in the body of the rat. The metabolites were primarily excreted in the feces and secondarily in the urine. Analysis of urinary metabolites containing the (14)C label, 6.0 hr following an iv dose, indicated the presence of highly polar neutral (53.27%) and acidic (39.23%) species.
PMID:2848334 Walter BA et al; Toxicol Appl Pharmacol 96 (2): 258-68 (1988)
Nonoxynol-9 interacts with the lipids in the membranes of the acrosome and the midpiece of the sperm. The sperm membranes are lysed; the acrosome, neck and midpiece of the spermatozoa are loosened and then detached which results in their immobilization and death.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TODAY
Dosage Form : SPONGE;VAGINAL
Dosage Strength : 1GM
Packaging :
Approval Date : 1983-04-01
Application Number : 18683
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : DELFEN
Dosage Form : AEROSOL;VAGINAL
Dosage Strength : 12.5%
Packaging :
Approval Date : 1982-01-01
Application Number : 14349
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Softgels
Grade : Oral
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Suspension, Tablet
Grade : Topical, Oral
Category : Coating Systems & Additives, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Thickeners and Stabilizers
Brand Name : Carboxymethyl Cellulose
Application : Coating Systems & Additives, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Thickeners and Stabilizers
Excipient Details : Carboxymethylcellulose serves as a disintegrant, binder, coating agent & thickener in oral formulations, also as a stabilizer in topical formulations.
Grade : Not Available
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : ReadiLYCOAT® D CLEAR 110.01
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Grade : Oral
Category : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Film Formers & Plasticizers, Rheology Modifiers
Brand Name : Carboxymethyl Cellulose
Application : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Film Formers & Plasticizers, Rheology Modifiers
Excipient Details : Carboxy Methyl Cellulose is used as a binder, disintegrant, film-former, and rheology modifier in the production of tablets and capsules.
Application : Coating Systems & Additives
Excipient Details : Novomix is used as a ready mix film coating system in the production of pharmaceutical tablets.
Dosage Form : Tablet
Grade : Not Available
Category : Coating Systems & Additives, Rheology Modifiers
Application : Coating Systems & Additives, Rheology Modifiers
Excipient Details : Tablet Coating, Rheology Modifier
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Carboxymethyl cellulose sodium
Grade : Not Available
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : ReadiLYCOAT® D CLEAR 110.01 MS
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Application : Coating Systems & Additives
Excipient Details : Film Coating
Application : Coating Systems & Additives
Excipient Details : Film Coating
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgels
Grade : Parenteral, Oral, Topical
Category : Film Formers & Plasticizers, Parenteral, Thickeners and Stabilizers, Topical
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Suspension, Tablet
Grade : Topical, Oral
Category : Coating Systems & Additives, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Thickeners and Stabilizers
Brand Name : Carboxymethyl Cellulose
Application : Coating Systems & Additives, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Thickeners and Stabilizers
Excipient Details : Carboxymethylcellulose serves as a disintegrant, binder, coating agent & thickener in oral formulations, also as a stabilizer in topical formulations.
Dosage Form : Cream / Lotion / Ointment, Emulsion, Injectable / Parenteral, Softgels, Tablet
Grade : Oral, Topical, Parenteral
Category : Film Formers & Plasticizers, Parenteral, Topical

Dosage Form : Capsule, Cream / Lotion / Ointment, Gel, Tablet
Grade : Topical, Oral
Category : Film Formers & Plasticizers, Lubricants & Glidants, Topical
Application : Taste Masking
Pharmacopoeia Ref : Not Available
Technical Specs : Pharma Grade/Food Grade
Ingredient(s) : Sorbitol
Grade : Not Available
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : ReadiLYCOAT® D CLEAR 110.01
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Market Place

Reply
29 Aug 2024
Reply
26 Jul 2022
Reply
12 Jun 2020
Reply
09 Dec 2019
Reply
09 Oct 2018

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?