
Synopsis
Synopsis
0
KDMF
0
VMF
0
Weekly News Recap #Phispers
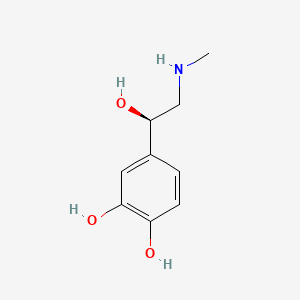

1. 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-benzenediol
2. Acetate, Epinephrine
3. Adrenaline
4. Adrenaline Acid Tartrate
5. Adrenaline Bitartrate
6. Adrenaline Hydrochloride
7. Epifrin
8. Epinephrine Acetate
9. Epinephrine Bitartrate
10. Epinephrine Hydrochloride
11. Epinephrine Hydrogen Tartrate
12. Epitrate
13. Lyophrin
14. Medihaler-epi
1. L-adrenaline
2. Adrenaline
3. L-epinephrine
4. 51-43-4
5. Adrenalin
6. Levoepinephrine
7. Nephridine
8. (-)-epinephrine
9. Adnephrine
10. Chelafrin
11. Epinefrina
12. Epinephran
13. Epirenan
14. Epipen
15. (-)-adrenaline
16. Adrenal
17. Epifrin
18. Renoform
19. Vasoconstrictine
20. Primatene Mist
21. Bronkaid Mist
22. Sus-phrine
23. Hypernephrin
24. Renostypticin
25. Supracapsulin
26. Supranephrane
27. Suprarenaline
28. Adrenalinum
29. Adrenine
30. Glauposine
31. Hemisine
32. Hemostasin
33. Hemostatin
34. Levorenin
35. Levorenine
36. Lyophrin
37. Mucidrina
38. Nieraline
39. Paranephrin
40. Renaglandin
41. Renaleptine
42. Renalina
43. Scurenaline
44. Simplene
45. Styptirenal
46. Takamina
47. Twinject
48. Vasotonin
49. Bosmin
50. Exadrin
51. Tonogen
52. (r)-adrenaline
53. Adrin
54. (-)-(r)-epinephrine
55. L-epirenamine
56. Antiasthmatique
57. Esphygmogenina
58. Methylarterenol
59. Supranephrine
60. Adrenamine
61. Adrenapax
62. Adrenasol
63. Adrenodis
64. Adrenohorma
65. Adrenosan
66. Adrenutol
67. Astmahalin
68. Astminhal
69. Balmadren
70. Bernarenin
71. Biorenine
72. Brevirenin
73. Bronkaid
74. Drenamist
75. Dylephrin
76. Glaucosan
77. Glycirenan
78. Haemostasin
79. Haemostatin
80. Hektalin
81. Hyporenin
82. Intranefrin
83. Isoptoepinal
84. Kidoline
85. Myosthenine
86. Sindrenina
87. Soladren
88. Sphygmogenin
89. Stryptirenal
90. Supradin
91. Supranefran
92. Supranol
93. Takamine
94. Tokamina
95. Vasodrine
96. Corisol
97. Glaucon
98. Mytrate
99. Suprel
100. Asthma-nefrin
101. Asmatane Mist
102. 4-[(1r)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol
103. L(-)-epinephrine
104. Sympathin I
105. (r)-epinephrine
106. Asthma Meter Mist
107. Epiglaufrin
108. Adrenalin-medihaler
109. Levorenen
110. Eppy
111. Dyspne-inhal
112. R-(-)-epinephrine
113. Adrenalin In Oil
114. Epinefrin [czech]
115. Epinephrinum
116. Epinefrin
117. Renostyptin
118. Surrenine
119. Susphrine
120. Citanest Forte
121. 1-epinephrine
122. 1-adrenalin
123. Epipen Jr.
124. Vaponefrin
125. (r)-(-)-adrenaline
126. Rcra Waste Number P042
127. L-epinephrine (synthetic)
128. Adrenalina [dcit]
129. Adrenatrate
130. Epinefrina [inn-spanish]
131. Epinephrinum [inn-latin]
132. Metanephrin
133. Renaglandulin
134. Renostypricin
135. Vasoconstrictor
136. Epirenin
137. Renagladin
138. Surenine
139. Vasoton
140. L-adrenalin
141. R-adrenaline
142. Suprarenin
143. Methylaminoethanolcatechol
144. Auvi-q
145. (r)-(-)-epinephrine
146. L-methylaminoethanolcatechol
147. D-adrenaline
148. Sus-phrine Sulfite-free
149. Lyodrin
150. Symjepi
151. (r)-(-)-adnephrine
152. L-epinehphrine
153. (r)-(-)-epirenamine
154. L-1-(3,4-dihydroxyphenyl)-2-methylaminoethanol
155. 4-[(1r)-1-hydroxy-2-(methylamino)ethyl]-1,2-benzenediol
156. (-)-3,4-dihydroxy-alpha-((methylamino)methyl)benzyl Alcohol
157. Rcra Waste No. P042
158. 1,2-benzenediol, 4-(1-hydroxy-2-(methylamino)ethyl)-, (r)-
159. Adrenalin (tn)
160. Micronefrin
161. Epitrate
162. Levo-methylaminoethanolcatechol
163. (-)-r-epinephrine
164. Racepinefrine, (r)-
165. Ai3-19015
166. Adrenalin Chloride
167. Sus-phrine Sulphite-free
168. Lopac-e-4642
169. 1,2-benzenediol, 4-[(1r)-1-hydroxy-2-(methylamino)ethyl]-
170. 1-1-(3,4-dihydroxyphenyl)-2-methylaminoethanol
171. Chembl679
172. Ykh834o4bh
173. Adrenan
174. Adrine
175. D-epinephrine
176. (r)-4-[1-hydroxy-2-(methylamino)ethyl]-1,2-benzenediol
177. D-epifrin
178. Chebi:28918
179. Benzyl Alcohol, 3,4-dihydroxy-alpha-((methylamino)methyl)-, (-)-
180. Asthmanefrin
181. Nsc-62786
182. 4-(1-hydroxy-2-(methylamino)ethyl)-1,2-benzenediol
183. Ncgc00015417-01
184. Ncgc00142615-03
185. Ncgc00142615-08
186. Adrenalina
187. 1,2-benzenediol, 4-((1r)-1-hydroxy-2-(methylamino)ethyl)-
188. 1,2-benzenediol, 4-(1-hydroxy-2-(methylamino)ethyl)-, (theta)-
189. Dsstox_cid_2986
190. Adrenaline (l-adrenaline)
191. Dsstox_rid_76819
192. Dsstox_gsid_22986
193. Rac Epinephrine
194. (-)-3,4-dihydroxy-a-[2-(methylamino)ethyl]benzyl Alcohol
195. Adrenaclick
196. Exadri
197. 1,2-benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (r)-
198. Epipen E Z Pen
199. L-epinephine
200. Asthmahaler Mist
201. Epi E Z Pen Jr
202. Ana-guard
203. Ana-kit
204. Cas-51-43-4
205. Epipen Ez Pen
206. L-adrenaline Base
207. (-)-adrenalin
208. Twinject 0.3
209. Epi Ez Pen Jr
210. Adrop
211. Epinephrine [usan:inn:jan]
212. Epipen Auto-injector
213. Ccris 4812
214. Twinject 0.15
215. Auvi-q Auto-injector
216. Epipen Jr
217. Hsdb 4289
218. Bronkaid Suspension Mist
219. Einecs 200-098-7
220. Nsc 62786
221. Epipen Jr. Auto-injector
222. Unii-ykh834o4bh
223. (r)-4-(1-hydroxy-2-(methylamino)ethyl)benzene-1,2-diol
224. Epinephrin
225. Levoreninum
226. Anapen
227. Nsc62786
228. 4-((1r)-1-hydroxy-2-(methylamino)ethyl)-1,2-benzenediol
229. Bupivacaine Hcl And Epinephrine
230. Benzyl Alcohol, 3,4-dihydroxy-.alpha.-[(methylamino)methyl]-, (-)-
231. Benzyl Alcohol, 3,4-dihydroxy-.alpha.-((methylamino)methyl)-, (-)-
232. Epinephrine [usp:inn:ban:jan]
233. Ale
234. Iop
235. Epinephrine (usp)
236. Epipen (tn)
237. Nchembio747-comp9
238. Twinject 0.30
239. Adrenaline/epinephrine
240. Adrenaline (jp15)
241. Adrenaline (jp17)
242. Epitrate (salt/mix)
243. Auvi-q (tn)
244. Adrenaline [jan]
245. Epinephrine (usp/inn)
246. Epinephrine [mi]
247. Epinephrine [inn]
248. Adrenalinum [hpus]
249. Epinephrine [hsdb]
250. Bmse000316
251. 4-[1-hydroxy-2-(methylamino)ethyl]-1,2-benzenediol #
252. Adrenaline [mart.]
253. Citanest Forte (salt/mix)
254. L-adrenaline (epinephrine)
255. Schembl3814
256. 3,4-dihydroxy-.alpha.-((methylamino)methyl)benzyl Alcohol
257. Epinephrine [usp-rs]
258. Epinephrine [who-dd]
259. Epinephrine [who-ip]
260. Gtpl479
261. Bidd:gt0119
262. E4250_sigma
263. Adrenaline [ep Impurity]
264. Dtxsid5022986
265. Bdbm44818
266. Cid_6852374
267. Hy-b0447b
268. Zinc39089
269. Adrenaline [ep Monograph]
270. Component Of E-pilo (salt/mix)
271. Epinephrine [orange Book]
272. Hms3884h06
273. Epinephrine [usp Monograph]
274. 104655-05-2
275. Bcp09042
276. Epinephrinum [who-ip Latin]
277. Tox21_111562
278. Bdbm50029050
279. Hsci1_000215
280. Pdsp1_001120
281. Pdsp2_001104
282. S2522
283. 51-43-4 (free Base)
284. Octocaine Component Epinephrine
285. Akos024283500
286. Iontocaine Component Epinephrine
287. Tox21_111562_1
288. Ccg-204593
289. D29a657
290. Db00668
291. Sdccgsbi-0050486.p002
292. Smp1_000227
293. (-)-epinephrine, >=97.0% (nt)
294. Epinephrine Component Of Octocaine
295. Ncgc00142615-01
296. Ncgc00142615-04
297. Ncgc00142615-05
298. Ncgc00142615-06
299. Ncgc00142615-07
300. Ncgc00142615-09
301. Ncgc00142615-18
302. Ac-13188
303. Ac-31211
304. As-13813
305. Epinephrine Component Of Iontocaine
306. 4,5-beta-trihydroxy-n-methylphenethylamine
307. A0173
308. Sw219274-1
309. (-)-epinephrine;l-adrenaline;(-)-adrenalin
310. C00788
311. D00095
312. D88222
313. Ab00573227-11
314. Ab00573227-12
315. Ab00573227-13
316. Ab00573227_14
317. Ab00573227_15
318. Q132621
319. 4-(1-hydroxy-2-methylamino-ethyl)benzene-1,2-diol
320. Q-200601
321. Sr-01000075267-8
322. Noradrenaline Tartrate Impurity A [ep Impurity]
323. 4-[(1r)-1-hydroxy-2-methylaminoethyl]benzene-1,2-diol
324. Lidosite Topical System Kit Component Epinephrine
325. Z2686405805
326. (-)-3,4-dihydroxy-a-[(methylamino)methyl]-benzyl Alcohol
327. Adrenaline, European Pharmacopoeia (ep) Reference Standard
328. Epinephrine Component Of Lidosite Topical System Kit
329. (-)-3,4-dihydroxy-alpha-[(methylamino)methyl]-benzyl Alcohol
330. (-)-3,4-dihydroxy-alpha-[2-(methylamino)ethyl]benzyl Alcohol
331. 4-[(1r)-1-hydroxy-2-(methylamino)ethyl]pyrocatechol;tartaric Acid
332. (-)-3,4-dihydroxy-.alpha.-((methylamino)methyl)benzyl Alcohol
333. Adrenaline With Impurity F, European Pharmacopoeia (ep) Reference Standard
334. 2,3-dihydroxybutanedioic Acid;4-[(1r)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol
335. 2,3-bis(oxidanyl)butanedioic Acid;4-[(1r)-2-(methylamino)-1-oxidanyl-ethyl]benzene-1,2-diol
| Molecular Weight | 183.20 g/mol |
|---|---|
| Molecular Formula | C9H13NO3 |
| XLogP3 | -1.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 183.08954328 g/mol |
| Monoisotopic Mass | 183.08954328 g/mol |
| Topological Polar Surface Area | 72.7 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 154 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 16 | |
|---|---|
| Drug Name | Adrenalin |
| PubMed Health | Epinephrine (Into the nose) |
| Drug Classes | Decongestant |
| Drug Label | Adrenalin (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL (1:1000) epinephrine, packaged as 1 mL of solution in a single-use clear glass vial or 30 mL of solution in a multiple-dose amber glass vial. In the 1... |
| Active Ingredient | Epinephrine hydrochloride |
| Dosage Form | Injectable |
| Route | Intramuscular, intraocular, subcutaneous; Intramuscular, subcutaneous |
| Strength | eq 1mg base/ml (eq 1mg base/ml); eq 30mg base/30ml (eq 1mg base/ml) |
| Market Status | Prescription |
| Company | Par Sterile Products |
| 2 of 16 | |
|---|---|
| Drug Name | Auvi-q |
| PubMed Health | Epinephrine (Injection) |
| Drug Classes | Anaphylaxis Agent, Anesthetic Adjunct, Bronchodilator, Vasopressor |
| Drug Label | AuviQ (epinephrine injection, USP) 0.3 mg and 0.15 mg is an auto-injector and a combination product containing drug and device components.AuviQ includes audible (electronic voice instructions, beeps) and visible (LED lights) cues for use.Ea... |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | eq 0.15mg/delivery; eq 0.3mg/delivery |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 3 of 16 | |
|---|---|
| Drug Name | Epinephrine |
| PubMed Health | Epinephrine |
| Drug Classes | Anaphylaxis Agent, Anesthetic Adjunct, Antiglaucoma, Bronchodilator, Decongestant, Vasopressor |
| Drug Label | Each EpiPen Auto-Injector delivers a single dose of 0.3 mg epinephrine injection, USP, 1:1000 (0.3 mL) in a sterile solution. Each EpiPen Jr Auto-Injector delivers a single dose of 0.15 mg epinephrine injection, USP, 1:2000 (0.3 mL) in a sterile... |
| Active Ingredient | Epinephrine hydrochloride |
| Dosage Form | Solution |
| Route | Iv (infusion) |
| Strength | eq 1mg base/ml (eq 1mg base/ml) |
| Market Status | Prescription |
| Company | Belcher Pharms |
| 4 of 16 | |
|---|---|
| Drug Name | Epipen |
| PubMed Health | Epinephrine |
| Drug Classes | Anaphylaxis Agent, Anesthetic Adjunct, Antiglaucoma, Bronchodilator, Decongestant, Vasopressor |
| Drug Label | Each EpiPen Auto-Injector delivers a single dose of 0.3 mg epinephrine injection, USP, 1:1000 (0.3 mL) in a sterile solution. Each EpiPen Jr Auto-Injector delivers a single dose of 0.15 mg epinephrine injection, USP, 1:2000 (0.3 mL) in a sterile... |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | 0.3mg/delivery |
| Market Status | Prescription |
| Company | Mylan Speclt |
| 5 of 16 | |
|---|---|
| Drug Name | Epipen jr. |
| Drug Label | EpiPen (epinephrine injection, USP) 0.3 mg and EpiPen Jr (epinephrine injection, USP) 0.15 mg are auto-injectors and combination products containing drug and device components.Each EpiPen Auto-Injector, 0.3 mg delivers a single dose of 0.3 mg epineph... |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | 0.15mg/delivery |
| Market Status | Prescription |
| Company | Mylan Speclt |
| 6 of 16 | |
|---|---|
| Drug Name | Septocaine |
| Active Ingredient | Articaine hydrochloride; epinephrine bitartrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.0085mg base/1.7ml (4%; eq 0.01mg base/ml); eq 0.017mg base/1.7ml (4%; 4%; eq 0.005mg base/ml) |
| Market Status | Prescription |
| Company | Deproco |
| 7 of 16 | |
|---|---|
| Drug Name | Twinject 0.15 |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | eq 0.15mg/delivery |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 8 of 16 | |
|---|---|
| Drug Name | Twinject 0.3 |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | eq 0.3mg/delivery |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 9 of 16 | |
|---|---|
| Drug Name | Auvi-q |
| PubMed Health | Epinephrine (Injection) |
| Drug Classes | Anaphylaxis Agent, Anesthetic Adjunct, Bronchodilator, Vasopressor |
| Drug Label | AuviQ (epinephrine injection, USP) 0.3 mg and 0.15 mg is an auto-injector and a combination product containing drug and device components.AuviQ includes audible (electronic voice instructions, beeps) and visible (LED lights) cues for use.Ea... |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | eq 0.15mg/delivery; eq 0.3mg/delivery |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 10 of 16 | |
|---|---|
| Drug Name | Epinephrine |
| PubMed Health | Epinephrine |
| Drug Classes | Anaphylaxis Agent, Anesthetic Adjunct, Antiglaucoma, Bronchodilator, Decongestant, Vasopressor |
| Drug Label | Each EpiPen Auto-Injector delivers a single dose of 0.3 mg epinephrine injection, USP, 1:1000 (0.3 mL) in a sterile solution. Each EpiPen Jr Auto-Injector delivers a single dose of 0.15 mg epinephrine injection, USP, 1:2000 (0.3 mL) in a sterile... |
| Active Ingredient | Epinephrine hydrochloride |
| Dosage Form | Solution |
| Route | Iv (infusion) |
| Strength | eq 1mg base/ml (eq 1mg base/ml) |
| Market Status | Prescription |
| Company | Belcher Pharms |
| 11 of 16 | |
|---|---|
| Drug Name | Epipen |
| PubMed Health | Epinephrine |
| Drug Classes | Anaphylaxis Agent, Anesthetic Adjunct, Antiglaucoma, Bronchodilator, Decongestant, Vasopressor |
| Drug Label | Each EpiPen Auto-Injector delivers a single dose of 0.3 mg epinephrine injection, USP, 1:1000 (0.3 mL) in a sterile solution. Each EpiPen Jr Auto-Injector delivers a single dose of 0.15 mg epinephrine injection, USP, 1:2000 (0.3 mL) in a sterile... |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | 0.3mg/delivery |
| Market Status | Prescription |
| Company | Mylan Speclt |
| 12 of 16 | |
|---|---|
| Drug Name | Epipen jr. |
| Drug Label | EpiPen (epinephrine injection, USP) 0.3 mg and EpiPen Jr (epinephrine injection, USP) 0.15 mg are auto-injectors and combination products containing drug and device components.Each EpiPen Auto-Injector, 0.3 mg delivers a single dose of 0.3 mg epineph... |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | 0.15mg/delivery |
| Market Status | Prescription |
| Company | Mylan Speclt |
| 13 of 16 | |
|---|---|
| Drug Name | Septocaine |
| Active Ingredient | Articaine hydrochloride; epinephrine bitartrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.0085mg base/1.7ml (4%; eq 0.01mg base/ml); eq 0.017mg base/1.7ml (4%; 4%; eq 0.005mg base/ml) |
| Market Status | Prescription |
| Company | Deproco |
| 14 of 16 | |
|---|---|
| Drug Name | Twinject 0.15 |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | eq 0.15mg/delivery |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 15 of 16 | |
|---|---|
| Drug Name | Twinject 0.3 |
| Active Ingredient | Epinephrine |
| Dosage Form | Injectable |
| Route | Intramuscular, subcutaneous |
| Strength | eq 0.3mg/delivery |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 16 of 16 | |
|---|---|
| Drug Name | Adrenalin |
| PubMed Health | Epinephrine (Into the nose) |
| Drug Classes | Decongestant |
| Drug Label | Adrenalin (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL (1:1000) epinephrine, packaged as 1 mL of solution in a single-use clear glass vial or 30 mL of solution in a multiple-dose amber glass vial. In the 1... |
| Active Ingredient | Epinephrine hydrochloride |
| Dosage Form | Injectable |
| Route | Intramuscular, intraocular, subcutaneous; Intramuscular, subcutaneous |
| Strength | eq 1mg base/ml (eq 1mg base/ml); eq 30mg base/30ml (eq 1mg base/ml) |
| Market Status | Prescription |
| Company | Par Sterile Products |
Adrenergic alpha-Agonists; Adrenergic beta-Agonists; Adrenergic Agonists; Bronchodilator Agents; Mydriatics; Sympathomimetics; Vasoconstrictor Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Epinephrine is the drug of choice in the emergency treatment of severe acute anaphylactic reactions including anaphylactic shock. Symptoms such as urticaria, pruritus, angioedema, and swelling of the lips, eyelids, and tongue which may result from reactions to drugs, sera, insect stings, food, or other allergens may be relieved by epinephrine. Epinephrine should be given to all patients with signs of systemic reactions, particularly hypotension, airway swelling, or definite breathing difficulty. Circulatory support during anaphylactic shock requires rapid volume resuscitation and vasopressor therapy to support blood pressure; epinephrine is the drug of choice for the treatment of both vasodilation/hypotension and cardiac arrest associated with anaphylaxis. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1389
Epinephrine may be added to solutions of some local anesthetics to decrease the rate of vascular absorption of the anesthetic, thereby localizing anesthesia and prolonging the duration of anesthesia; the risk of systemic toxicity from the local anesthetic is also decreased. Epinephrine may be applied topically to control superficial bleeding from arterioles or capillaries in the skin, mucous membranes, or other tissues. Bleeding from larger vessels is not controllable by topical application of epinephrine. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1390
Epinephrine is used for its a-adrenergic stimulatory effects to increase blood flow in advanced cardiovascular life support (ACLS) during cardiopulmonary resuscitation (CPR). The principal beneficial effects of the drug in patients with cardiac arrest result from increases in aortic diastolic blood pressure and in myocardial and cerebral blood flow during resuscitation. The value and safety of the beta-adrenergic effects of epinephrine are controversial because they may increase myocardial work and reduce subendocardial perfusion. Epinephrine remains a drug of choice and a high priority for ACLS in cardiac arrest to facilitate return of spontaneous circulation. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1389
For more Therapeutic Uses (Complete) data for EPINEPHRINE (15 total), please visit the HSDB record page.
Epinephrine should not be used in cardiogenic shock because it increases myocardial oxygen demand, nor should it be used in hemorrhagic or traumatic shock.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1389
Vet: epinephrine injection (1:1000): do not use in acute hypotension produced by phenothiazine derived tranquilizers, since further depression of blood pressure can occur. Do not use when cyclopropane or halogenated anesthetics are used because of possible cardiac collapse. Do not use in treatment of vascular shock. Do not use in patients known to be sensitive to epinephrine ... Use with caution in hyperthyroid animals; animals being treated with thyroid, digitalis, or mercurial diuretics. Do not use injection if it is brown or contains a precipitate.
Aronson, C.E. (ed.). Veterinary Pharmaceuticals & Biologicals, 1980-1981. Media, Pa.: Harwal Publishing Co., 1980., p. 16-48
A prospective study where topical epinephrine was used on burn and non-burn patients and five patients served as controls without epinephrine usage. Catecholamine concentrations were measured and to estimate the systemic effects of epinephrine, serum lactate and pyruvate concentrations were analyzed and perioperative haemodynamic changes recorded. Compared to the baseline values, there was a significant increase in the heart rate, serum epinephrine and lactate concentrations and LP-ratios in the burn patients and an increase in the epinephrine concentrations in the non-burn patients at 1 and 2 h. Epinephrine and lactate concentrations and LP-ratios were also higher in the burn patients compared to the other groups. Altogether, there were no changes in the control group. This study showed that the use of topical epinephrine has systemic effects on hemodynamics and serum epinephrine concentrations. Increased epinephrine concentrations in burn patients suggest increased absorption properties in these patients. The increased lactate concentrations and LP-ratios suggest tissue ischaemia, likely in skin.
PMID:19481869 Papp AA et al; Burns 35 (6): 832-9 (2009).
Some manufacturers state that epinephrine is contraindicated for parenteral use during the second stage of labor; parenteral administration of the drug to maintain blood pressure during spinal anesthesia for delivery can cause acceleration of fetal heart rate and should not be used in obstetric patients when maternal systolic/diastolic blood pressure exceeds 130/80 mm Hg. Epinephrine should be administered cautiously by oral inhalation to pregnant patients. Epinephrine should be used during pregnancy only if the potential benefits justify the possible risks to the fetus. There is some evidence that epidural administration of lidocaine with epinephrine during labor is safe.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1393
For more Drug Warnings (Complete) data for EPINEPHRINE (21 total), please visit the HSDB record page.
The minimum lethal human dose by subcutaneous injection is estimated as 4 mg.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 548
Epinephrine injection is indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitos), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis. Injectable epinephrine is intended for immediate/urgent administration in patients, who are found to be at increased risk for anaphylaxis, including individuals with a history of anaphylaxis. Selection of the appropriate dosage strength is determined according to body weight. Epinephrine's cardiac effects may be of use in restoring cardiac rhythm in cardiac arrest due to various causes but is not used in cardiac failure or in hemorrhagic, traumatic, or cardiogenic shock. Epinephrine is used as a hemostatic agent. It is also used in treating mucosal congestion of hay fever, rhinitis, and acute sinusitis; to relieve bronchial asthmatic paroxysms; in syncope due to complete heart block or carotid sinus hypersensitivity; for symptomatic relief of serum sickness, urticaria, angioneurotic edema; for resuscitation in cardiac arrest following anesthetic accidents; in simple (open angle) glaucoma; for relaxation of uterine musculature and to inhibit uterine contractions. Epinephrine injection can be utilized to prolong the action of local anesthetics. In addition to the above, epinephrine is used as an over the counter (OTC) agent for the intermittent symptoms of asthma, such as wheezing, tightness of chest and shortness of breath. It is also used for the maintenance of mydriasis during intraocular surgery.
FDA Label
Epinephrine is a sympathomimetic drug. It causes an adrenergic receptive mechanism on effector cells and mimics all actions of the sympathetic nervous system except those on the facial arteries and sweat glands. Important effects of epinephrine include increased heart rate, myocardial contractility, and renin release via beta-1 receptors. Beta-2 effects produce bronchodilation which may be useful as an adjunct treatment of asthma exacerbations as well as vasodilation, tocolysis, and increased aqueous humor production. In croup, nebulized epinephrine is associated with both clinically and statistically significant transient reduction of croup symptoms 30 minutes post-treatment. Epinephrine also alleviates pruritus, urticaria, and angioedema and may be helpful in relieving gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxing effects on the smooth muscle of the stomach, intestine, uterus, and urinary bladder.
Adrenergic alpha-Agonists
Drugs that selectively bind to and activate alpha adrenergic receptors. (See all compounds classified as Adrenergic alpha-Agonists.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
Adrenergic beta-Agonists
Drugs that selectively bind to and activate beta-adrenergic receptors. (See all compounds classified as Adrenergic beta-Agonists.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Mydriatics
Agents that dilate the pupil. They may be either sympathomimetics or parasympatholytics. (See all compounds classified as Mydriatics.)
C01CA24
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AD - Other agents for local oral treatment
A01AD01 - Epinephrine
B - Blood and blood forming organs
B02 - Antihemorrhagics
B02B - Vitamin k and other hemostatics
B02BC - Local hemostatics
B02BC09 - Epinephrine
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CA - Adrenergic and dopaminergic agents
C01CA24 - Epinephrine
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AA - Sympathomimetics, plain
R01AA14 - Epinephrine
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03A - Adrenergics, inhalants
R03AA - Alpha- and beta-adrenoreceptor agonists
R03AA01 - Epinephrine
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EA - Sympathomimetics in glaucoma therapy
S01EA01 - Epinephrine
Absorption
Following I.V. (intravenous) injection, epinephrine disappears rapidly from the blood stream. Subcutaneously or I.M. (intramuscular) administered epinephrine has a rapid onset and short duration of action. Subcutaneous (SC) administration during asthmatic attacks may produce bronchodilation within 5 to 10 minutes, and maximal effects may occur within 20 minutes. The drug becomes fixed in the tissues rapidly,.
Route of Elimination
The majority of the dose of epinephrine is seen excreted in the urine,. About 40% of a parenteral dose of epinephrine is excreted in urine as metanephrine, 40% as VMA, 7% as 3-methoxy-4-hydroxyphenoglycol, 2% as 3,4-dihydroxymandelic acid, and the rest as acetylated derivatives. These metabolites are excreted mainly as the sulfate conjugates and, to a lesser extent, the glucuronide conjugates. Only small amounts of the drug are excreted completely unchanged.
Clearance
Intravenous injection produces an immediate and intensified response. Following intravenous injection, epinephrine disappears rapidly from the blood stream.
Following topical application of radiolabeled epinephrine to the eye in rabbits, highest concentrations of the drug in tissues and fluids other than the eye occurred in the pituitary gland, with lower concentrations in the intestine, fat, adrenal gland, kidney, heart, lung, spleen, ovary, pancreas, liver, uterus, muscle, brain, and serum. In humans, systemically absorbed epinephrine crosses the placenta but not the blood-brain barrier. Systemically absorbed epinephrine distributes into milk.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2926
Epinephrine is not effective after oral admin because it is rapidly conjugated and oxidized in GI mucosa and liver. Absorption from sc tissues occurs slowly because of local vasoconstriction ... Absorption is more rapid after im than after sc injection ... Epinephrine is rapidly inactivated in the body.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 247
In a prospective, randomized, five-way crossover study in rabbits, ... plasma epinephrine concentrations /were measured/ before, and at intervals up to 180 min after epinephrine administration by intramuscular or subcutaneous injection, or by inhalation, with intravenous epinephrine and intramuscular saline as the positive and negative controls, respectively. Maximum plasma epinephrine concentrations were higher, and occurred more rapidly, after intramuscular injection than after subcutaneous injection or inhalation, and were 7719+/-3943 (S.E.M.) pg/mL at 32.5+/-6.6 min, 2692+/-863 pg/mL at 111.7+/-30.8 min and 1196+/-369 pg/mL at 45. 8+/-19.2 min, respectively. Intravenous injection of epinephrine resulted in a plasma concentration of 3544+/-422 pg/mL at 5 min, and an elimination half-life (t(1/2)) of 11.0+/-2.5 min. In the saline control study, the endogenous epinephrine concentration peaked at 518+/-142 pg/mL. CONCLUSION: In this model, absorption of epinephrine was significantly faster after intramuscular injection than after subcutaneous injection or inhalation. The extent of absorption was satisfactory after both intramuscular and subcutaneous injections. Neither the rate nor the extent of absorption was satisfactory after administration by inhalation.
PMID:10870098 Gu X et al; Biopharm Drug Dispos 20 (8): 401-5 (1999).
3 groups of 5 greyhounds received 1.5 ug/kg epinephrine 1:200,000 in either lidocaine 0.5%, bupivacaine 0.5% or 0.9% saline. Dogs were anesthetized and 40% of the allocated epinephrine solution was infiltrated beneath the perianal skin and each of the 4 quadrants of the rectal mucosa was injected with the remainder of the solution. Plasma epinephrine, lidocaine, bupivacaine, lactate, glucose and potassium concn were measured at 1, 2, 5, 10 and 30 min following infiltration. Peak plasma epinephrine concn were recorded 2 min following rectal mucosal infiltration in all 3 groups. Plasma epinephrine concn were significantly higher (p < 0.01) in the lidocaine group at 1 and 2 min following infiltration. Both plasma bupivacaine and lidocaine peaked 10 min after infiltration and thereafter tended to decr towards baseline concn. Plasma bupivacaine concn were significantly higher (p < 0.01) than plasma lidocaine concn throughout the study period. There were no significant differences in metabolic or biochemical indices within or between the 3 groups. However, both plasma glucose and lactate concn were elevated and peaked 10 min after infiltration, while plasma potassium concn remained unchanged throughout the study period. Heart rate in the bupivacaine group was significantly reduced at 30 min following infiltration (p < 0.05). There were no significant differences observed in the mean arterial and pulse pressures among the 3 groups.
PMID:2758538 Flynn N et al; Can J Anaesth 36 (4): 397-401 (1989)
Epinephrine is well absorbed after subcutaneous or IM injection; absorption can be hastened by massaging the injection site. Both rapid and prolonged absorption occur after subcutaneous injection of the longer-acting aqueous suspension (no longer commercially available in the US). Epinephrine also is absorbed following endotracheal administration, although serum concentrations achieved may be only 10% of those with an equivalent IV dose.. After oral inhalation of epinephrine in the usual dosage, absorption is slight and the effects of the drug are restricted mainly to the respiratory tract. Absorption increases somewhat when larger doses are inhaled, and systemic effects may occur.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1394
Epinephrine is rapidly inactivated mainly by enzymic transformation to metanephrine or normetanephrine, either of which is then conjugated and excreted in the urine in the form of both sulfates and glucuronides. Either sequence results in the formation of 3-methoxy-4- hydroxy-mandelic acid(vanillylmandelic acid, VMA) which is shown to be detectable in the urine. Epinephrine is rapidly inactivated in the body mostly by the enzymes COMT (catechol-O-methyltransferase) and MAO (monoamine oxidase). The liver is abundant in the above enzymes, and is a primary, although not essential, tissue in the degradation process.
The pharmacologic actions of epinephrine are terminated mainly by uptake and metabolism in sympathetic nerve endings. Circulating drug is metabolized in the liver and other tissues by a combination of reactions involving the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). The major metabolites are metanephrine and 3-methoxy-4-hydroxymandelic acid (vanillylmandelic acid, VMA) both of which are inactive. About 40% of a parenteral dose of epinephrine is excreted in urine as metanephrine, 40% as VMA, 7% as 3-methoxy-4-hydroxyphenoglycol, 2% as 3,4-dihydroxymandelic acid, and the remainder as acetylated derivatives. These metabolites are excreted mostly as the sulfate conjugates and, to a lesser extent, the glucuronide conjugates. Only small amounts of the drug are excreted unchanged.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1394
Circulating epinephrine is metabolized in the liver and is taken up into adrenergic neurons and metabolized by MAO and catechol-O-methyltransferase to metadrenaline, sulfate conjugates, and hydroxy derivatives of mandelic acid.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 548
Epinephrine has known human metabolites that include Epinephrine sulfate.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The plasma half-life is approximately 2-3 minutes. However, when administered by subcutaneous or intramuscular injection, local vasoconstriction may delay absorption so that epinephrine's effects may last longer than the half-life suggests.
Elimination half life is 1 minute.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 548
Epinephrine acts on alpha and beta-adrenergic receptors. Epinephrine acts on alpha and beta receptors and is the strongest alpha receptor activator. Through its action on alpha-adrenergic receptors, epinephrine minimizes the vasodilation and increased the vascular permeability that occurs during anaphylaxis, which can cause the loss of intravascular fluid volume as well as hypotension. Epinephrine relaxes the smooth muscle of the bronchi and iris and is a histamine antagonist, rendering it useful in treating the manifestations of allergic reactions and associated conditions. This drug also produces an increase in blood sugar and increases glycogenolysis in the liver. Through its action on beta-adrenergic receptors, epinephrine leads to bronchial smooth muscle relaxation that helps to relieve bronchospasm, wheezing, and dyspnea that may occur during anaphylaxis.
The mechanism of rise in blood pressure ... is threefold: a direct myocardial stimulation that increases the strength of ventricular contraction (positive inotropic action), an increased heart rate (positive chronotropic action), vasoconstriction in many vascular beds, especially in precapillary resistance vessels of skin, mucosa, and kidney, along with marked constriction of veins.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 243
... Epinephrine affects respiration primarily by relaxing bronchial muscle. It has a powerful bronchodilator action, most evident when bronchial muscle is contracted because of disease, as in bronchial asthma, or in response to drugs or various autacoids. In such situations, epinephrine has a striking therapeutic effect as a physiological antagonist to substances that cause bronchoconstriction. The beneficial effects of epinephrine in asthma also may arise from inhibition of antigen-induced release of inflammatory mediators from mast cells, and to a lesser extent from diminution of bronchial secretions and congestion within the mucosa. Inhibition of mast cell secretion is mediated by beta2 receptors, while the effects on the mucosa are mediated by alpha receptors
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 246
The electrophysiologic effects of circulating epinephrine in humans were examined in four study groups of 10 subjects each. In 10 subjects without structural heart disease (Group 1) and in 10 patients with coronary disease or dilated cardiomyopathy (Group 2) epinephrine infusion at 25 and 50 ng/kg body weight per min for 14 min resulted in an elevation of the plasma epinephrine concentration in the physiologic range. In both groups it produced a dose-dependent decrease in the effective refractory period of the atrium, atrioventricular node and ventricle and improvement in atrioventricular node conduction. Epinephrine facilitated the induction of sustained ventricular tachycardia in 3 of the 20 subjects. In Group 3, a beta-adrenergic blocking dose of propranolol was added to the infusion of 50 ng/kg per min of epinephrine. Propranolol not only reversed the effects of epinephrine, but also lengthened these variables compared with baseline values. In group 4, propranolol was administered first, followed by 50 ng/kg per min of epinephrine. Propranolol alone slowed atrioventricular node conduction and mildly prolonged the refractory periods. In the presence of beta-blockade, epinephrine had no effect on atrioventricular node properties but resulted in a lengthening of the atrial and ventricular effective refractory periods. In conclusion, epinephrine in physioloic doses shortens the effective refractory period of the atrium, atrioventricular node and ventricle, improves atrioventricular node conduction and may facilitate the induction of sustained ventricular tachycardia. The overall electrophysiologic effects of epinephrine result from stimulation of beta-receptors. Stimulation of alpha-receptors by epinephrine has no effect on the atrioventricular node but prolongs the effective refractory period of the atrium and ventricle, partially offsetting the shortening of refractory periods mediated by beta-receptor stimulation.
PMID:2835408 Morady F et al; J Am Coll Cardiol 11 (6): 1235-44 (1988)
Epinephrine plays a key role in the control of vasomotor tone; however, the participation of the NO/cGMP pathway in response to beta-adrenoceptor activation remains controversial. To evaluate the involvement of the endothelium in the vascular response to epinephrine, we assessed NO production, endothelial NO synthase phosphorylation, and tissue accumulation of cGMP in the perfused arterial mesenteric bed of rat. Epinephrine elicited a concentration-dependent increase in NO (EC(50) of 45.7 pM), which was coupled to cGMP tissue accumulation. Both NO and cGMP production were blocked by either endothelium removal (saponin) or NO synthase inhibition (N(omega)-nitro-L-arginine). Blockade of beta(1)- and beta(2)-adrenoceptors with 1 microM propranolol or beta(3)-adrenoceptor with 10 nM SR 59230A displaced rightward the concentration-NO production curve evoked by epinephrine. Selective stimulation of beta(1)-, beta(2)-, or beta(3)-adrenoceptors also resulted in NO and cGMP production. Propranolol (1 microM) inhibited the rise in NO induced by isoproterenol or the beta(2)-adrenoceptor agonists salbutamol, terbutaline, or fenoterol. Likewise, 10 nM SR 59230A reduced the effects of the beta(3)-adrenoceptor agonists BRL 37344, CGP 12177, SR 595611A, or pindolol. The NO production induced by epinephrine and BRL 37344 was associated with the activation of the phosphatidylinositol 3-kinase/Akt pathway and phosphorylation of eNOS in serine 1177. In addition, in anaesthetized rats, bolus administration of isoproterenol, salbutamol, or BRL 37344 produced NO-dependent reductions in systolic blood pressure. These findings indicate that beta(1)-, beta(2)-, and beta(3)-adrenoceptors are coupled to the NO/cGMP pathway, highlighting the role of the endothelium in the vasomotor action elicited by epinephrine and related beta-adrenoceptor agonists.
PMID:19429833 Figueroa XF et al; Am J Physiol Heart Circ Physiol 297 (1): H134-43 (2009)
 Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.
Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2018-01-11
Pay. Date : 2016-12-28
DMF Number : 30997
Submission : 2016-09-20
Status : Active
Type : II

Certificate Number : R1-CEP 2016-232 - Rev 00
Issue Date : 2023-02-09
Type : Chemical
Substance Number : 2303
Status : Valid
Date of Issue : 2018-07-04
Valid Till : 2022-01-15
Written Confirmation Number : TFDA-00021
Address of the Firm :
 Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25874
Submission : 2012-03-12
Status : Active
Type : II

NDC Package Code : 59349-0019
Start Marketing Date : 2018-11-07
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21213
Submission : 2007-12-31
Status : Active
Type : II
Date of Issue : 2020-10-16
Valid Till : 2022-07-21
Written Confirmation Number : WC-0075a3
Address of the Firm :


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-01-05
Pay. Date : 2021-12-30
DMF Number : 28617
Submission : 2014-09-18
Status : Active
Type : II

Date of Issue : 2022-08-08
Valid Till : 2025-07-14
Written Confirmation Number : WC-0168
Address of the Firm :


GDUFA
DMF Review : Reviewed
Rev. Date : 2021-06-09
Pay. Date : 2021-04-30
DMF Number : 35384
Submission : 2020-12-16
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-12-05
Pay. Date : 2023-09-29
DMF Number : 38733
Submission : 2023-09-27
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-11-13
Pay. Date : 2014-06-13
DMF Number : 25989
Submission : 2012-04-13
Status : Active
Type : II

Certificate Number : R1-CEP 2013-266 - Rev 01
Issue Date : 2023-01-31
Type : Chemical
Substance Number : 2303
Status : Valid

Registration Number : 226MF10196
Registrant's Address : Via Curiel 34, 20067 Paulo, Milano, ITALY
Initial Date of Registration : 2014-10-27
Latest Date of Registration : --

NDC Package Code : 12828-0082
Start Marketing Date : 2012-08-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : It is used as a filler in formulation.
Pharmacopoeia Ref : Not Available
Technical Specs : Also Available as Comprecel M102, M102CG, M103, M105, M124, M113, M200, M224, M301, M302
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : It is used as a lubricant.
Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : It is used as a filler in formulation.
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : It is used as a lubricant.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Excipient Details : It is used as a filler in formulation.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Reply
28 Dec 2024
Reply
30 Jul 2024
Reply
26 Jun 2024
Reply
07 Jun 2024
Reply
16 Mar 2024
Reply
18 Nov 2023
Reply
09 Sep 2023
Reply
17 Jul 2023
Reply
07 Jun 2023
Reply
17 Mar 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?