
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. 4-chlorophenol Ion (1+)
2. 4-chlorophenol, Copper (+1) Salt
3. 4-chlorophenol, Nickel (+2) Salt
4. 4-chlorophenol, Potassium Salt
5. 4-chlorophenol, Sodium Salt
6. 4-chlorophenol, Titanium (+4) Salt
7. 4-chlorophenoxide
8. 4-monochlorophenol
9. P-chlorophenol
10. Para-monochlorophenol
11. Parachlorophenol
1. 106-48-9
2. P-chlorophenol
3. Parachlorophenol
4. Phenol, 4-chloro-
5. 4-hydroxychlorobenzene
6. Phenol, P-chloro-
7. Applied 3-78
8. 4-monochlorophenol
9. P-chlorophenic Acid
10. P-chlorfenol
11. 4-chlorphenol
12. 4-chloro-1-hydroxybenzene
13. P-chlorfenol [czech]
14. 4-chloro-phenol
15. Nsc 2877
16. Mfcd00002318
17. Parachlorophenol (jan/usp)
18. 3dlc36a01x
19. Chebi:28078
20. Nsc-2877
21. Ncgc00090814-01
22. Dsstox_cid_1871
23. Dsstox_rid_76376
24. Dsstox_gsid_21871
25. P-chlorophenol, Liquid [un2021] [keep Away From Food]
26. P-chlorophenol, Solid [un2020] [keep Away From Food]
27. Parachlorophenol [usp]
28. Cas-106-48-9
29. Ccris 642
30. Hsdb 1414
31. Einecs 203-402-6
32. Unii-3dlc36a01x
33. 4chlorophenol
34. P-chloro Phenol
35. Ai3-19422
36. Para-chlorophenol
37. Parachloro Phenol
38. 4-chloro Phenol
39. 4ch
40. Parachlorophenol,(s)
41. P-chlorophenol, Solid
42. P-chlorophenol, Liquid
43. Spectrum_000939
44. Spectrum2_000968
45. Spectrum3_000539
46. Spectrum4_000468
47. Spectrum5_001228
48. Wln: Qr Dg
49. 4-clc6h4oh
50. Bmse000461
51. Ec 203-402-6
52. 4-chlorophenol, >=99%
53. P-chlorophenol [mi]
54. Schembl28864
55. Bspbio_002157
56. Kbiogr_000916
57. Kbioss_001419
58. Mls002454432
59. Bidd:er0011
60. Chembl57053
61. Divk1c_000310
62. P-chlorophenol [inci]
63. Parachlorophenol [jan]
64. Spectrum1500460
65. Spbio_000975
66. Parachlorophenol [hsdb]
67. Zinc1885
68. Parachlorophenol [vandf]
69. 4-chlorphenol [who-dd]
70. Dtxsid1021871
71. Parachlorophenol [mart.]
72. Bdbm36299
73. Hms500p12
74. Kbio1_000310
75. Kbio2_001419
76. Kbio2_003987
77. Kbio2_006555
78. Kbio3_001657
79. Nsc2877
80. Parachlorophenol [usp-rs]
81. Ninds_000310
82. Hms1920f08
83. Hms2091n08
84. Hms2230b13
85. Hms3373o02
86. Pharmakon1600-01500460
87. Tox21_111028
88. Tox21_201704
89. Tox21_302860
90. Ccg-40184
91. Nsc757263
92. Stl194293
93. 4-chlorophenol, For Synthesis, 98%
94. Akos000118967
95. Tox21_111028_1
96. Db13154
97. Nsc-757263
98. Idi1_000310
99. Parachlorophenol [usp Monograph]
100. Ncgc00090814-02
101. Ncgc00090814-03
102. Ncgc00090814-04
103. Ncgc00090814-05
104. Ncgc00090814-07
105. Ncgc00256497-01
106. Ncgc00259253-01
107. 4-chlorophenol 10 Microg/ml In Methanol
108. Smr001252242
109. 4-chlorophenol 100 Microg/ml In Methanol
110. Sbi-0051474.p003
111. Db-028832
112. 4-chlorophenol, Puriss., >=99.0% (gc)
113. Cs-0005709
114. Ft-0618238
115. 4-chlorophenol 100 Microg/ml In Isopropanol
116. 4-chlorophenol 1000 Microg/ml In Isopropanol
117. C02124
118. D00149
119. Ab00052066_06
120. 4-chlorophenol, Pestanal(r), Analytical Standard
121. Sr-05000001691
122. J-001599
123. Q2179668
124. Sr-05000001691-1
125. Brd-k40992116-001-05-5
126. F0001-0124
127. Z1262246118
128. Parachlorophenol, United States Pharmacopeia (usp) Reference Standard
129. 4-chlorophenol Solution, 725 Mg/l In H2o, For Aox Determination (according To Din 38409-h14)
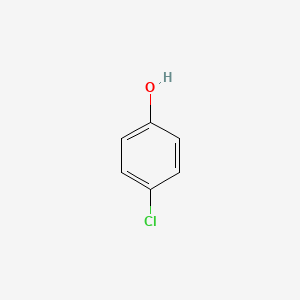
| Molecular Weight | 128.55 g/mol |
|---|---|
| Molecular Formula | C6H5ClO |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 128.0028925 g/mol |
| Monoisotopic Mass | 128.0028925 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 66.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Local antibacterial agent in root canal therapy
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1111
Topical antiseptic /cat/
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 359
Medication (Vet): /Formerly/ as topical antiseptic in ointments
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 106
Used as an intermediate in organic synthesis of dyes and drugs. Local antibacterial agent in root canal therapy, as topical antiseptic in ointments
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Absorption
absorbed from gastrointestinal tract.
Route of Elimination
87% of 4-chlorophenol was excreted in urine of dogs as sulfate and glucuronide.
A number of rabbit studies have shown that metabolism of the monochlorophenols is principally via conjugation. In one study, groups of 6 rabbits were treated by gavage with 171.3 mg/kg of 2-CP or 4-CP emulsified in water as a single dose. For both isomers, the 24-hour urine analysis indicated that between 78.1 and 88.3% of the administered dose was excreted as the glucuronide, and between 12.8 and 20.6% of the administered dose was excreted as the ethereal sulfate. A total of 101.7 and 101.1% of the administered 2-CP or 4-CP doses, respectively, was accounted for as urinary glucuronide and sulfate conjugates.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Chlorophenols PB/99/166639 p.80 (July 1999). Available from, as of October 28, 2008: https://www.atsdr.cdc.gov/toxpro2.html#
Metabolism was ... investigated in 4 rabbits, each treated by gavage with an average dose of 395 mg/kg/day of 4-CP. After 36 hours, 54.1% of the administered dose appeared in the urine as the glucuronide conjugate, and 10.4% of the administered dose appeared in the ethereal sulfate fraction. Only 0.1% of the administered dose was excreted as 4-chlorocatechol. The low total recovery (64.5%) in the latter experiment limits conclusions.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Chlorophenols PB/99/166639 p.80 (July 1999). Available from, as of October 28, 2008: https://www.atsdr.cdc.gov/toxpro2.html#
In a limited study in dogs about half of an oral dose of 2- or 4-CP was excreted in the urine as the ethereal sulfate. No evidence for metabolism to mercapturic acid was found.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Chlorophenols PB/99/166639 p.80 (July 1999). Available from, as of October 28, 2008: https://www.atsdr.cdc.gov/toxpro2.html#
... Absorbed from ... gastrointestinal tract & ... parenteral sites of injection. ... Excreted as conjugates of sulfuric & glucuronic acid. ... Urine darkens after standing /Chlorophenols/
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1615-16
P-Chlorophenol yields p-chloroanisole in guinea pigs. P-Chlorophenol yields 4-chlorocatechol p-chloro phenyl-beta-D-glucuronide & p-chlorphenyl sulfate in rabbits. P-Chlorophenol yields p-chlorophenyl sulfate in rats.
P-Chlorophenol yields p-chloroanisole in guinea pigs.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-36
P-Chlorophenol yields 4-chlorocatechol p-chloro phenyl-beta-D-glucuronide & p-chlorphenyl sulfate in rabbits.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-36
P-Chlorophenol yields p-chlorophenyl-beta-D-glucuronide * p-chlorophenyl sulfate in hens.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-36
P-Chlorophenol yields p-chlorophenyl sulfate in rats.
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-36
For more Metabolism/Metabolites (Complete) data for 4-CHLOROPHENOL (10 total), please visit the HSDB record page.
4-Chlorophenol has known human metabolites that include (2S,3S,4S,5R)-6-(4-Chlorophenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The major mode of action of chlorophenols appears to be the uncoupling of oxidative phosphorylation. The strength of the uncoupling effect is related to the degree of chlorination: PCP is the strongest inhibitor of oxidative phosphorylation, MCP the weakest. To a lesser extent, inhibition of oxidative phosphorylation is affected by the positions of the chlorine atoms on the molecule.
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
80
PharmaCompass offers a list of 4-Chlorophenol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right 4-Chlorophenol manufacturer or 4-Chlorophenol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred 4-Chlorophenol manufacturer or 4-Chlorophenol supplier.
PharmaCompass also assists you with knowing the 4-Chlorophenol API Price utilized in the formulation of products. 4-Chlorophenol API Price is not always fixed or binding as the 4-Chlorophenol Price is obtained through a variety of data sources. The 4-Chlorophenol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 4-Chlorophenol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 4-Chlorophenol, including repackagers and relabelers. The FDA regulates 4-Chlorophenol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 4-Chlorophenol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 4-Chlorophenol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 4-Chlorophenol supplier is an individual or a company that provides 4-Chlorophenol active pharmaceutical ingredient (API) or 4-Chlorophenol finished formulations upon request. The 4-Chlorophenol suppliers may include 4-Chlorophenol API manufacturers, exporters, distributors and traders.
click here to find a list of 4-Chlorophenol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
4-Chlorophenol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 4-Chlorophenol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 4-Chlorophenol GMP manufacturer or 4-Chlorophenol GMP API supplier for your needs.
A 4-Chlorophenol CoA (Certificate of Analysis) is a formal document that attests to 4-Chlorophenol's compliance with 4-Chlorophenol specifications and serves as a tool for batch-level quality control.
4-Chlorophenol CoA mostly includes findings from lab analyses of a specific batch. For each 4-Chlorophenol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
4-Chlorophenol may be tested according to a variety of international standards, such as European Pharmacopoeia (4-Chlorophenol EP), 4-Chlorophenol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (4-Chlorophenol USP).
