
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
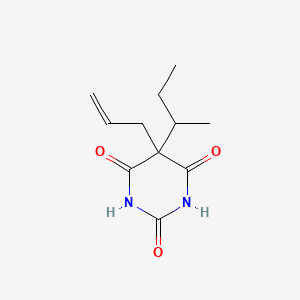

1. 5-allyl-5-sec-butylbarbituric Acid
2. Allyl-sec-butyl-barbituric Acid
1. Lotusate
2. Profundol
3. 115-44-6
4. 5-allyl-5-sec-butylbarbituric Acid
5. Talbutal [inn]
6. Sec-butyl Allyl Barbituric Acid
7. 5-allyl-5-(1-methylpropyl) Barbituric Acid
8. Win 5095
9. Talbutal (inn)
10. Barbituric Acid, 5-allyl-5-sec-butyl-
11. 5-(1-methylpropyl)-5-(2-propenyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
12. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methylpropyl)-5-(2-propenyl)-
13. 4yir8202ax
14. Talbumalum
15. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methypropyl)-5-(2-propenyl)-
16. Talbutale [dcit]
17. Talbutale
18. Talbutalum
19. Talbutalum [inn-latin]
20. Lotusate (tn)
21. Hsdb 3397
22. Talbutal [usp:inn]
23. 5-butan-2-yl-5-prop-2-enyl-1,3-diazinane-2,4,6-trione
24. Einecs 204-090-4
25. Unii-4yir8202ax
26. Latusate
27. Talbutal [hsdb]
28. Talbutal [mi]
29. Talbutal [vandf]
30. Talbutal Ciii(250 Mg)
31. Dsstox_cid_3630
32. Talbutal [mart.]
33. Talbutal [who-dd]
34. Dsstox_rid_97553
35. Dsstox_gsid_23630
36. Schembl122147
37. Talbutal [orange Book]
38. Chembl1200802
39. Dtxsid8023630
40. Chebi:134923
41. Tox21_113715
42. Hy-u00276
43. 5-(1-methylpropyl)-5-prop-2-en-1-ylpyrimidine-2,4,6(1h,3h,5h)-trione
44. Cs-7222
45. Db00306
46. Ncgc00253567-01
47. Cas-115-44-6
48. Ft-0674795
49. D06887
50. Q409942
51. 5-allyl-5-(1-methylpropyl)-barbituric Acid
52. 5-allyl-5-sec-butyl-2,4,6(1h,3h,5h)-pyrimidinetrione
53. 5-allyl-5-sec-butylpyrimidine-2,4,6(1h,3h,5h)-trione
54. (rs)-5-allyl-5-sec-butylpyrimidine-2,4,6(1h,3h,5h)-trione
55. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(1-methylpropyl)-5-(2-propen-1-yl)-
| Molecular Weight | 224.26 g/mol |
|---|---|
| Molecular Formula | C11H16N2O3 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 224.11609238 g/mol |
| Monoisotopic Mass | 224.11609238 g/mol |
| Topological Polar Surface Area | 75.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 329 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
"SHORT- TO INTERMEDIATE-ACTING" COMPOUNDS ARE USED PRINCIPALLY AS SEDATIVE-HYPNOTIC AGENTS. /BARBITURATES, SHORT- TO INTERMEDIATE-ACTING/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 117
BARBITURATE WITH INTERMEDIATE DURATION OF ACTION. FOLLOWING USUAL HYPNOTIC DOSE, ONSET OF SLEEP OCCURS IN 15-30 MIN & LASTS FROM 6-8 HR. DOSE--USUAL HYPNOTIC, 120 MG BEFORE BEDTIME; SEDATIVE, 30 MG 2 OR 3 TIMES DAILY.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1003
BARBITURATES MAY BE USED FOR PREANESTHETIC MEDICATION & TO PRODUCE BASAL ANESTHESIA. ... BARBITURATES ARE EMPLOYED AS DIAGNOSTIC & THERAPEUTIC AIDS IN PSYCHIATRY, IN NARCOANALYSIS & NARCOTHERAPY. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 119
The primary Medication Classification of the US Veterans Administration is CN301: Barbituric Acid Derivatives, Sedatives/Hypnotics.
United States Pharmacopeial Convention; USP dispensing Information 12th ed Vol IA p.573 (1992)
For more Therapeutic Uses (Complete) data for TALBUTAL (6 total), please visit the HSDB record page.
EVALUATION OF EFFECTS OF GENERAL DEPRESSANTS ON FETUS & NEONATES IS DIFFICULT, & PLACE THAT BARBITURATES SHOULD OCCUPY IN OBSTETRICS IS STILL CONTROVERSIAL. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 119
CAPACITY OF BARBITURATES TO INCR SYNTHESIS OF PORPHYRINS IS RESPONSIBLE FOR ONE ... BIZARRE & DANGEROUS SIDE EFFECT. IN PT SUFFERING FROM ACUTE INTERMITTENT PORPHYRIA, DRUGS MAY PPT SEVERE ATTACK, POSSIBLY ... PARALYSIS & DEATH. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 111
WHEN RENAL FUNCTION IS IMPAIRED, BARBITURATES THAT DEPEND ON KIDNEY FOR ELIMINATION MAY CAUSE SEVERE CNS DEPRESSION & ... FURTHER REDUCE RENAL FUNCTION. ... UREMIA MAY INCR SENSITIVITY TO BARBITURATES ... /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 115
... CIRRHOTIC PT MAY SHOW INCR SENSITIVITY TO HYPNOTICS ... BARBITURATES SHOULD BE ADMIN WITH CAUTION, & INITIALLY IN REDUCED DOSES, TO PT WITH HEPATIC DAMAGE. ... SHOULD NOT BE ADMIN TO PT SHOWING PREMONITORY SIGNS OF HEPATIC COMA. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 115
For more Drug Warnings (Complete) data for TALBUTAL (15 total), please visit the HSDB record page.
For use as a sedative and hypnotic.
Talbutal is a short to intermediate-acting barbiturate that is a nonselective central nervous system (CNS) depressant. As with other barbiturates, talbutal is capable of producing all levels of CNS mood alteration from excitation to mild sedation, hypnosis, and deep coma. Barbiturates may also induce anesthesia at sufficiently high therapeutic doses.
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CA - Barbiturates, plain
N05CA07 - Talbutal
THERE EXISTS NO IMPENETRABLE BARRIER TO DIFFUSION OF BARBITURATES IN BODY ... SMALL AMT ... MAY APPEAR IN MILK AFTER INGESTION OF LARGE DOSES. ... READILY CROSS PLACENTAL BARRIER, &, WITHIN FEW MIN AFTER INJECTION OF SHORT-... ACTING BARBITURATE, CONCN IN FETAL BLOOD APPROACHES THAT IN MATERNAL VENOUS BLOOD. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 112
THREE MOST IMPORTANT FACTORS AFFECTING DISTRIBUTION & FATE OF BARBITURATES ARE LIPID SOLUBILITY ... PROTEIN BINDING, & EXTENT OF IONIZATION. ... THE MORE HIGHLY LIPID-SOL COMPD ARE SHORT-ACTING AGENTS... THEY TEND TO BE MORE RAPIDLY DEGRADED METABOLICALLY & ARE ALMOST COMPLETELY REABSORBED BY RENAL TUBULE. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 113
For sedative hypnotic use, the barbiturates are usually administered orally. Such doses are rapidly and probably completely absorbed; sodium salts are absorbed more rapidly than the corresponding free acids, especially from liquid formulations. The onset of action varies from 10 to 60 min, depending upon the agent and the formulation, and is delayed by the presence of food in stomach. /Barbiturates/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 361
Barbiturates are distributed widely and readily cross the placenta. Binding to plasma proteins is a function of lipid solubility and is greatest for thiopental, which is bound to the extent of 65% or more. The highly lipid soluble barbiturates, led by those used to induce anesthesia, undergo redistribution after intravenous injection. Uptake into less vascular tissue, especially muscle and fat, leads to a decline in the concentration of barbiturate in the plasma and brain.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 361
Elimination/% excreted unchanged: renal/trace
United States Pharmacopeial Convention; USP Dispensing Information 12 th ed Vol IA p. 588 (1992)
MOST BARBITURATES ARE TRANSFORMED IN BODY TO INACTIVE METABOLITES. PRINCIPAL SITE OF BIOTRANSFORMATION IS LIVER. OXYBARBITURATES ARE TRANSFORMED ONLY BY LIVER; THIOBARBITURATES MAY BE TRANSFORMED TO SMALL EXTENT IN OTHER TISSUES, ESP KIDNEY & BRAIN ... /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 115
Hepatic biotransformation, primarily by the hepatic microsomal enzyme system. /Barbiturates/
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IA p.575 (1992)
With the exception of the less lipid-soluble aprobarbital and phenobarbital, nearly complete metabolism and/or conjugation of barbiturates in the liver precedes their renal excretion. The oxidation of radicals at C5 is the most important biotransformation responsible for termination of biological activity. Oxidation results in the formation of alcohols, kentones, phenols, or carboxylic acids, which may appear in the urine as such or as glucuronic acid conjugates. In some instances (e.g., phenobarbital), N-glucosylation is an important metabolic pathway. Other biotransformations include N-hydroxylation, desulfuration of thiobarbiturates to oxybarbiturates, opening of the barbituric acid ring, and N-dealkylation of N-alkylbarbiturates to active metabolites (e.g., mephobarbital to phenobarbital).
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 361
Half-life 15 hr
United States Pharmacopeial Convention; USP Dispensing Information 12 ed Vol. IA p. 588 (1992)
Talbutal binds to GABAA receptors at a distinct binding site associated with a Cl- ionopore of the receptor. Upon binding, talbutal increases the duration of time for which the Cl- ionopore is open, leading to prolonged inhibitory effect of GABA at the postsynaptic thalamic neuron.
BARBITURATES REVERSIBLY DEPRESS ACTIVITY OF ALL EXCITABLE TISSUES. NOT ALL TISSUES ARE AFFECTED @ SAME DOSE OR CONCN; CNS IS EXQUISITELY SENSITIVE, SO THAT WHEN BARBITURATES ARE GIVEN IN SEDATIVE OR HYPNOTIC DOSES, VERY LITTLE EFFECT ON SKELETAL, CARDIAC, OR SMOOTH MUSCLE OCCURS. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 104
... RETICULAR ACTIVATING SYSTEM IS EXQUISITELY SENSITIVE TO DEPRESSANT EFFECTS OF BARBITURATES ... WHATEVER ... EFFECTS OF BARBITURATES ELSEWHERE IN CNS, IT IS EFFECT ON RETICULAR SYSTEM THAT SEEMS TO BE RESPONSIBLE FOR INABILITY TO MAINTAIN WAKEFULNESS UNDER INFLUENCE OF BARBITURATE. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 108
BARBITURATES ARE RESP DEPRESSANTS, AFFECTING BOTH DRIVE TO RESP & MECHANISM RESPONSIBLE FOR RHYTHMIC CHARACTER OF RESP MOVEMENT. /BARBITURATES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 108
The mechanism(s) of action of the barbiturates is not completely known. Although the drugs act throughout the CNS, a site of particular sensitivity is the polysynaptic midbrain reticular formation which is concerned with the arousal mechanism. Barbiturates induce an imbalance in central inhibitory and facilitatory mechanisms influencing the cerebral cortex and the reticualar formation. The significance of the effect of barbiturates on neurotransmitters is unclear. It appears that the drugs decrease the excitability of both presynaptic and postsynaptic membranes. It has not been determined which of the various actions of barbiturates at cellular and synaptic levels are responsible for their sedative and hypnotic effects. /Barbiturates/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1317
For more Mechanism of Action (Complete) data for TALBUTAL (9 total), please visit the HSDB record page.
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?