
Synopsis
Synopsis
0
VMF
0
FDF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Evista
2. Keoxifene
3. Keoxifene Hydrochloride
4. Ly 139481
5. Ly 156758
6. Ly-139481
7. Ly-156758
8. Ly139481
9. Ly156758
10. Raloxifene
11. Raloxifene Hcl
1. 82640-04-8
2. Raloxifene Hcl
3. Evista
4. Keoxifene Hydrochloride
5. Keoxifene
6. Optruma
7. Raloxifene Teva
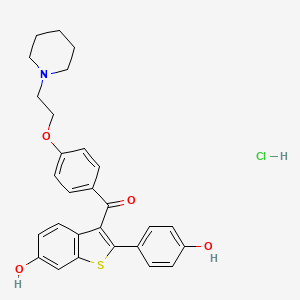
8. (6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl)(4-(2-(piperidin-1-yl)ethoxy)phenyl)methanone Hydrochloride
9. Raloxifene (hydrochloride)
10. Ly 156758
11. Ly156758
12. Ly-156758
13. Nsc-706725
14. 2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethoxy]phenyl}carbonyl)-1-benzothiophen-6-ol Hydrochloride
15. Chebi:50740
16. Evista (raloxifene Hydrochloride)
17. 4f86w47br6
18. 6-hydroxy-2-(p-hydroxyphenyl)benzo(b)thien-3-yl-p-(2-piperidinoethoxy)phenyl Ketone, Hydrochloride
19. Dsstox_cid_14181
20. Dsstox_rid_79119
21. Dsstox_gsid_34181
22. [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone Hydrochloride
23. [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone;hydrochloride
24. Smr000058508
25. Raloxifene Hydrochloride [usan]
26. Ncgc00015889-05
27. Cas-82640-04-8
28. C28h27no4s.hcl
29. Loxifen
30. Unii-4f86w47br6
31. Sr-01000076102
32. Cdt-raloxifene
33. Raloxifene-d4 Hcl
34. Raloxifene Cloridrato
35. Evista (tn)
36. Mfcd01938233
37. Prestwick_1035
38. Raloxifene Hydrochloride [usan:usp]
39. Methanone, Hydrochloride
40. Cloridrato De Raloxifeno
41. Clorhidrato De Raloxifeno
42. Chlorhydrate De Raloxifene
43. Ly139481 Hydrochloride
44. Ly156758 Hydrochloride
45. Chembl1116
46. Schembl19077
47. Mls000859902
48. Mls001332533
49. Mls001332534
50. Mls002222293
51. Raloxifene Hydrochloride, Solid
52. Evista, Raloxifene Hydrochloride
53. Raloxifene Hydrochloride- Bio-x
54. Dtxsid1034181
55. Ly156758 (keoxifene) Hcl
56. Ly-139481 Hcl
57. Hms1570n05
58. Pharmakon1600-01505622
59. Amy23426
60. Bcp05713
61. Raloxifene Hydrochloride (jan/usp)
62. Tox21_110255
63. Tox21_302369
64. Tox21_501051
65. Hy-13738a
66. Nsc706725
67. Nsc759285
68. Raloxifene Hydrochloride [mi]
69. S1227
70. Raloxifene Hydrochloride [jan]
71. Akos008131940
72. Tox21_110255_1
73. Ac-8390
74. Ccg-213497
75. Cs-1775
76. Ds-2162
77. Ks-1102
78. Lp01051
79. Nc00665
80. Nsc 706725
81. Nsc 759285
82. Nsc-759285
83. Raloxifene Hydrochloride [mart.]
84. Raloxifene Hydrochloride [vandf]
85. Ncgc00015889-03
86. Ncgc00015889-11
87. Ncgc00092353-01
88. Ncgc00092353-03
89. Ncgc00094334-01
90. Ncgc00094334-02
91. Ncgc00255153-01
92. Ncgc00261736-01
93. Raloxifene Hydrochloride [usp-rs]
94. Raloxifene Hydrochloride [who-dd]
95. Br164310
96. Methanone, (6-hydroxy-2-(4-hydroxyphenyl)benzo(b)thien-3-yl)(4-(2-(1-piperidinyl)ethoxy)phenyl)-, Hydrochloride
97. Methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-,hydrochloride (1:1)
98. Raloxifene Hydrochloride [ema Epar]
99. Eu-0101051
100. Ft-0630912
101. R0109
102. Sw197106-5
103. En300-52517
104. Raloxifene Hydrochloride [orange Book]
105. D02217
106. R 1402
107. Raloxifene Hydrochloride [ep Monograph]
108. Raloxifene Hydrochloride [usp Impurity]
109. Raloxifene Hydrochloride [usp Monograph]
110. 640r048
111. A840401
112. Q-201656
113. Sr-01000076102-2
114. Sr-01000076102-9
115. Q27122215
116. (6-oxo-1,6-dihydro-pyridazin-3-yloxy)-aceticacid
117. Raloxifene Hydrochloride, European Pharmacopoeia (ep) Reference Standard
118. Raloxifene Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
119. Raloxifene Hydrochloride, United States Pharmacopeia (usp) Reference Standard
120. (6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl)-(4-(2-(piperidin-1-yl)ethoxy)phenyl)methanone Hydrochloride
121. (6-oh-2-(4-oh-ph)benzo[b]thiophen-3-yl)(4-(2-(piperidin-1-yl)ethoxy)ph)methanone Hydrochloride
122. [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothien-3-yl][4-(2-piperidin-1-ylethoxy)phenyl]methanone Hydrochloride
123. [6-hydroxy-2-(4-hydroxyphenyl)-benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-methanone Hydrochloride
124. [6-hydroxy-2-(4-hydroxyphenyl)benzo [b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-methanone Hydrochloride
125. [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-methanone Hydrochloride
126. [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-methanone, Monohydrochloride
127. [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone Hydrochloride
128. [6-hydroxy-2[4-hydroxyphenyl)benzo [b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone Hydrochloride
129. [6-hydroxy-2[4-hydroxyphenyl)benzo [b]thien3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone Hydrochloride
130. [6-hydroxy-2[4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone Hydrochloride
131. 1-[2-(4-{[6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]carbonyl}phenoxy)ethyl]piperidinium Chloride
132. 6-hydroxy-2-(4-hydroxyphenyl)-3-[ 4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene, Hydrochloride
133. 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]-benzo[b]-thiophene Hydrochloride
134. 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]-benzo[b]thiophene Hydrochloride
135. 6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl 4-[2-(1-piperidinyl)ethoxy]phenyl Methanone Hydrochloride
136. 6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl 4-[2-(1-piperidinyl)ethoxy]phenyl Ketone Hydrochloride
137. 6-hydroxy-2-(p-hydroxyphenyl)benzo(.beta.)thien-3-yl-p-(2-piperidinoethoxy)phenyl Ketone, Hydrochloride
138. Methanone, (6-hydroxy-2-(4-hydroxyphenyl)benzo(.beta.)thien-3-yl)(4-(2-(1-piperidinyl)ethoxy)phenyl)-, Hydrochloride
139. Methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-, Hydrochloride (1:1)
140. Raloxifene Hydrochloride For Peak Identification, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 510.0 g/mol |
|---|---|
| Molecular Formula | C28H28ClNO4S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 509.1427572 g/mol |
| Monoisotopic Mass | 509.1427572 g/mol |
| Topological Polar Surface Area | 98.2 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 655 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Evista |
| PubMed Health | Raloxifene (By mouth) |
| Drug Classes | Antineoplastic Agent, Endocrine-Metabolic Agent |
| Drug Label | EVISTA (raloxifene hydrochloride) is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM) that belongs to the benzothiophene class of compounds. The chemical structure is:The chemical designation is m... |
| Active Ingredient | Raloxifene hydrochloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 60mg |
| Market Status | Prescription |
| Company | Lilly |
| 2 of 4 | |
|---|---|
| Drug Name | Raloxifene hydrochloride |
| Drug Label | EVISTA (raloxifene hydrochloride) is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM) that belongs to the benzothiophene class of compounds. The chemical structure is:The chemical designation is m... |
| Active Ingredient | Raloxifene hydrochloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 60mg |
| Market Status | Tentative Approval; Prescription |
| Company | Teva Pharms Usa; Invagen Pharms; Watson Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Evista |
| PubMed Health | Raloxifene (By mouth) |
| Drug Classes | Antineoplastic Agent, Endocrine-Metabolic Agent |
| Drug Label | EVISTA (raloxifene hydrochloride) is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM) that belongs to the benzothiophene class of compounds. The chemical structure is:The chemical designation is m... |
| Active Ingredient | Raloxifene hydrochloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 60mg |
| Market Status | Prescription |
| Company | Lilly |
| 4 of 4 | |
|---|---|
| Drug Name | Raloxifene hydrochloride |
| Drug Label | EVISTA (raloxifene hydrochloride) is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM) that belongs to the benzothiophene class of compounds. The chemical structure is:The chemical designation is m... |
| Active Ingredient | Raloxifene hydrochloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 60mg |
| Market Status | Tentative Approval; Prescription |
| Company | Teva Pharms Usa; Invagen Pharms; Watson Labs |
Optruma is indicated for the treatment and prevention of osteoporosis in post-menopausal women. A significant reduction in the incidence of vertebral, but not hip fractures has been demonstrated. When determining the choice of Optruma or other therapies, including oestrogens, for an individual postmenopausal woman, consideration should be given to menopausal symptoms, effects on uterine and breast tissues, and cardiovascular risks and benefits (see section 5. 1).
Evista is indicated for the treatment and prevention of osteoporosis in post-menopausal women. A significant reduction in the incidence of vertebral, but not hip fractures has been demonstrated. When determining the choice of Evista or other therapies, including oestrogens, for an individual postmenopausal woman, consideration should be given to menopausal symptoms, effects on uterine and breast tissues, and cardiovascular risks and benefits.
Raloxifene is indicated for the treatment and prevention of osteoporosis in postmenopausal women. A significant reduction in the incidence of vertebral, but not hip fractures has been demonstrated.
When determining the choice of raloxifene or other therapies, including oestrogens, for an individual postmenopausal woman, consideration should be given to menopausal symptoms, effects on uterine and breast tissues, and cardiovascular risks and benefits.
Selective Estrogen Receptor Modulators
A structurally diverse group of compounds distinguished from ESTROGENS by their ability to bind and activate ESTROGEN RECEPTORS but act as either an agonist or antagonist depending on the tissue type and hormonal milieu. They are classified as either first generation because they demonstrate estrogen agonist properties in the ENDOMETRIUM or second generation based on their patterns of tissue specificity. (Horm Res 1997;48:155-63) (See all compounds classified as Selective Estrogen Receptor Modulators.)
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
G03XC01
G03XC01
G03XC01
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2013-04-02
Pay. Date : 2013-03-22
DMF Number : 16143
Submission : 2002-09-23
Status : Active
Type : II

Certificate Number : R1-CEP 2014-054 - Rev 01
Issue Date : 2023-03-31
Type : Chemical
Substance Number : 2375
Status : Valid

Date of Issue : 2022-06-15
Valid Till : 2025-06-16
Written Confirmation Number : WC-0034
Address of the Firm :

Registrant Name : Alvogen Korea Co., Ltd.
Registration Date : 2020-09-17
Registration Number : 20200917-209-J-745
Manufacturer Name : Dr. Reddy's Laboratories Limited
Manufacturer Address : Chemical Technical Operations-Unit-II Plot No. 1, 75A, 75B, 105, 110, 111 & 112 Sri Venkateswara Co-operative Industrial Estate Bollaram, Jinnaram Mandal, Sangareddy District, Telangana, 502325 India

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21650
Submission : 2008-05-22
Status : Active
Type : II

NDC Package Code : 62704-0115
Start Marketing Date : 1998-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-04-29
Pay. Date : 2014-02-24
DMF Number : 27969
Submission : 2014-03-17
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-03-15
Pay. Date : 2013-09-11
DMF Number : 27064
Submission : 2013-05-06
Status : Active
Type : II

Date of Issue : 2022-09-01
Valid Till : 2025-07-02
Written Confirmation Number : WC-0074
Address of the Firm :

NDC Package Code : 65691-0079
Start Marketing Date : 2013-05-06
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2017-09-14
Pay. Date : 2017-08-01
DMF Number : 31952
Submission : 2017-08-23
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36445
Submission : 2021-10-23
Status : Active
Type : II

Certificate Number : CEP 2022-165 - Rev 00
Issue Date : 2024-04-22
Type : Chemical
Substance Number : 2375
Status : Valid

Date of Issue : 2022-09-19
Valid Till : 2025-07-02
Written Confirmation Number : WC-0119
Address of the Firm :

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26492
Submission : 2012-09-27
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21327
Submission : 2008-02-11
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
About the Company : Aasraw Biochemical Technology Co.,ltd was reorganized by a Shanghai based Biochemical Engineering Laboratory in 2008, which was built by 5 Chinese Ph. Doctors, who are majored at C...

About the Company : Founded in 1986 by Mr. P.V. Ramaprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born off a vision. The company comme...

About the Company : Cipla is a global pharmaceutical company whose goal is ensuring no patient shall be denied access to high quality & affordable medicine and support. Mission: Cipla’s mission i...

About the Company : ERREGIERRE, a family company, was founded in 1974 and today is one of the leading Italian active pharmaceutical ingredients manufactures for human and veterinary use. In ERREGIE...

About the Company : Medilux Laboratories has been providing quality back-end support to the pharmaceutical industry since our inception in 1988. Our resources are dedicated to promoting better health ...

About the Company : We produce Active Pharmaceutical Ingredients (APIs) providing integrated full-service capabilities. Olon’s ability to develop and manufacture Active Pharmaceutical Ingredients (...

About the Company : SAI-TECH PHARMACEUTICALS PRIVATE LIMITED is dedicated Active Pharmaceutical Ingredients (API) manufacturing company for producing & providing high quality products as per customer ...

About the Company : Established in 2000, Shodhana Laboratories offers a wide range of APIs and intermediates and caters to the generic and custom requirements of some of the most prestigious pharma co...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
17 Jul 2021
Reply
03 Apr 2021
Reply
18 Sep 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?