

1. 156-s
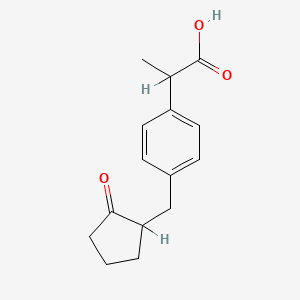
2. 2-(4-((2-oxocyclopentyl)methyl)phenyl)propionic Acid
3. 2-ocppp
4. Cs 600
5. Cs-600
6. Loxoprofen Alcohol
7. Loxoprofen Sodium Dihydrate
8. Loxoprofen Sodium, (r*,s*)-isomer
9. Sodium 2-(4-(2-oxocyclopentylmethyl)phenyl)propionate Dihydrate
10. Sodium Loxoprofen
1. 68767-14-6
2. 2-(4-((2-oxocyclopentyl)methyl)phenyl)propanoic Acid
3. Loxoprofene
4. Loxoprofeno
5. Koloxo
6. Loxoprofen [inn]
7. Loxoprofenum
8. Sodium Loxoprofen
9. Loxoprofen Sodium
10. 2-[4-[(2-oxocyclopentyl)methyl]phenyl]propanoic Acid
11. 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoic Acid
12. Loxoprofen Acid
13. Mfcd00864331
14. Chembl19299
15. 3583h0gzap
16. Loxoprofen Sodium Hydrate
17. Chebi:76172
18. Loxoprofen (inn)
19. Ncgc00015594-02
20. Dsstox_cid_25164
21. Dsstox_rid_80714
22. Dsstox_gsid_45164
23. Loxoprofene [french]
24. Loxoprofenum [latin]
25. 2-(4-((2-oxocyclopentyl)methyl)-phenyl)propanoic Acid
26. Loxoprofeno [spanish]
27. Cas-68767-14-6
28. Unii-3583h0gzap
29. Loxoprofen, Solid
30. Alpha-methyl-4-[(2-oxocyclopentyl)methyl]benzeneacetic Acid
31. Loxoprofen [mi]
32. Loxoprofen [who-dd]
33. Lopac0_000677
34. Schembl24423
35. (+-)-p-((2-oxocyclopentyl)methyl)hydratropic Acid
36. Dtxsid1045164
37. Hms3262g15
38. Hms3885n16
39. Loxoprofen (low-melting Polymorph)
40. Bcp10971
41. Hy-b0578
42. Loxoprofen (high-melting Polymorph)
43. Tox21_110178
44. Tox21_500677
45. Bdbm50140320
46. S4682
47. Akos015906359
48. Tox21_110178_1
49. Ac-8108
50. Ccg-204763
51. Db09212
52. Gs-3193
53. Lp00677
54. Sdccgsbi-0050656.p002
55. Ncgc00015594-03
56. Ncgc00015594-04
57. Ncgc00015594-08
58. Ncgc00094037-01
59. Ncgc00094037-02
60. Ncgc00261362-01
61. Ncgc00263577-01
62. Ac-15776
63. Sy113439
64. Db-055188
65. Eu-0100677
66. Ft-0641207
67. L0244
68. Cs-600; Cs600; Cs 600
69. D08149
70. L 0664
71. (+-)-((2-oxocyclopentyl)methyl)hydratropic Acid
72. 767l146
73. A836244
74. Sr-01000075955
75. Q-201324
76. Q-300011
77. Q2759348
78. Sr-01000075955-1
79. 2-[4-[(2-oxocyclopentyl)methyl]phenyl]propionic Acid
80. 2-[4-(2-oxo-cyclopentylmethyl)-phenyl]-propionic Acid
81. 2-[4-(2-oxocyclopentan-1-ylmethyl)phenyl]propionic Acid
82. (+/-)-p-((2-oxocyclopentyl)methyl)hydratropic Acid
83. 2-[4-(2-oxocyclopentan-1-ylmethyl)phenyl]-propionic Acid
84. 2-(4-((2-oxocyclopentan-1-yl)methyl)phenyl)propionic Acid
85. Benzeneacetic Acid, .alpha.-methyl-4-[(2-oxocyclopentyl)methyl]-
| Molecular Weight | 246.30 g/mol |
|---|---|
| Molecular Formula | C15H18O3 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 246.125594432 g/mol |
| Monoisotopic Mass | 246.125594432 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 316 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Loxoprofen is non-steroidal anti-inflammatory medication (NSAID) indicated for pain and inflammation related to musculoskeletal and joint disorders. In addition to its effects on pain, it is an antipyretic and anti-inflammatory medication.
Loxoprofen is a non-selective inhibitor of cyclooxygenase enzymes, which are responsible for the formation of various biologically active pain, fever, and inflammatory mediators. These include prostaglandins, prostacyclin, thromboxane, and arachidonic acid.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA31 - Loxoprofen
Absorption
Loxoprofen is rapidly and completely absorbed from the GI tract with a bioavailability of 95%. The absorption phase of the medication occurs in the first 4-6 hours after ingestion. Food ingestion with the medication causes a slight decrease in the rate of loxoprofen absorption.
Route of Elimination
50% renal excretion. This drug is 20% - 30% excreted in the stool.
Volume of Distribution
Loxoprofen has a volume of distribution of 0.16 L/kg.
Clearance
Most of the drug as unchanged loxoprofen, 6-0-desmethyl loxoprofen (less than 1%) and glucuronide or other conjugates (66-92%). In patients with renal failure, metabolites may accumulate.
Loxoprofen is a prodrug that is rapidly converted to its active trans-alcohol metabolite by carbonyl reductase in the liver. This same process also results in a cis-alcohol metabolite, though this isomer carries little pharmacological activity. The parent drug has also been observed to undergo oxidation via CYP3A4/5 to two hydroxylated metabolites (M3 and M4) and glucuronidation by UGT2B7 to two glucuronide metabolites (M5 and M6). The alcohol metabolites of loxoprofen also undergo glucuronide conjugation via UGT2B7 to two glucuronide metabolites (M7 and M8) prior to excretion. When applied in topical formulations, loxoprofen is metabolized to its active trans-alcohol form by carbonyl reductase in the skin.
The elimination half-life of Loxoprofen is approximately 15 hours. Steady concentration is achieved after 2-3 doses.
Loxoprofen itself is a prodrug and carries little-to-no pharmacological activity - it is rapidly metabolized to its trans-alcohol form, which is a potent and non-selective inhibitor of cyclooxygenase. Cyclooxygenase (COX) is present in 2 forms, COX-1 and COX-2, with each serving different functions. COX-1 is present in human cells and is constitutively released, performing cellular housekeeping functions such as mucus production and platelet aggregation. COX-2 is induced in human cells post-injury or due to other stimuli, is triggered to appear in large quantities at the sites of injury/stimuli, and is ultimately responsible for the mediation of inflammation and pain. Loxoprofen's active metabolite inhibits both COX isoforms, resulting in reduced expression of several mediators of pain, inflammation, and fever (e.g. prostaglandins, prostacyclin, thromboxane, etc).
BUILDING BLOCK

