
Synopsis
Synopsis
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
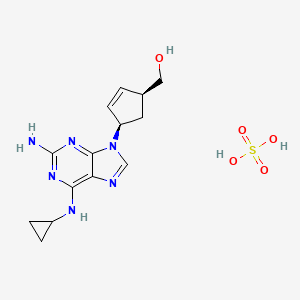

1. ((1r,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-cyclopent-2-enyl)methanol
2. ((1r,4s)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)cyclopent-2-enyl)methanol
3. (+)-abacavir
4. (+)-abacavir Sulfate
5. (+-)-abacavir Sulfate
6. (-)-cis-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol
7. (1r,4r)-abacavir
8. (1r,4s)-abacavir Sulfate
9. (1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol
10. (1s,4r)-4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-2-cyclopentene-1-methanol Succinate (1:1) (salt)
11. 1592u89
12. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, (1r,4s)-, Sulfate (2:1)
13. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, (1s,4r)-, Rel-, Sulfate (2:1)
14. 2-cyclopentene-1-methanol, 4-(2-amino-6-(cyclopropylamino)-9h-purin-9-yl)-, Hydrochloride, Hydrate (1:1:1), (1s,4r)-
15. Abacavir
16. Abacavir Enantiomer
17. Abacavir Hydrochloride Monohydrate
18. Abacavir Succinate
19. Abacavir Sulfate
20. Abacavir Sulfate Racemic
21. Abacavir Sulfate, (+)-
22. Abacavir Sulfate, (+-)-
23. Abacavir Sulfate, (1r,4s)-
24. Abacavir Sulphate
25. Abacavir, (+)-
26. Abacavir, (1r,4s)-
27. Abacavir, Trans-
28. Abacavir, Trans-, (+-)-
29. Abamune
30. Avacavir
31. Drg 0257
32. Drg-0257
33. Drg0257
34. Trans-abacavir
35. Trans-abacavir R,r-
1. Abacavir Sulfate
2. Abacavir (sulfate)
3. 216699-07-9
4. Abacavir (monosulfate)
5. 4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]-(1s,4r)-2-cyclopentene-1-methanolsulfate
6. (1s,4r)-4-[2-amino-6-(cyclopropylamino)-9h-purin-9-yl]-2-cyclopentene-1-methanol, Monosulfate
7. Schembl40818
8. Chembl1200619
9. Hy-17423b
10. Akos015994684
11. Ks-1221
12. Cs-0083626
| Molecular Weight | 384.41 g/mol |
|---|---|
| Molecular Formula | C14H20N6O5S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 4 |
| Exact Mass | 384.12158893 g/mol |
| Monoisotopic Mass | 384.12158893 g/mol |
| Topological Polar Surface Area | 185 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 496 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Abacavir sulfate |
| Drug Label | Abacavir sulfate is a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). A... |
| Active Ingredient | Abacavir sulfate |
| Dosage Form | Solution; Tablet |
| Route | oral; Oral |
| Strength | 300mg; 60mg; 60 mg; 20mg; eq 300mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Hetero Labs Ltd Iii; Mylan Pharms; Aurobindo Pharma; Cipla; Matrix Labs; Apotex; Strides Arcolab |
| 2 of 2 | |
|---|---|
| Drug Name | Abacavir sulfate |
| Drug Label | Abacavir sulfate is a synthetic carbocyclic nucleoside analogue with inhibitory activity against HIV-1. The chemical name of abacavir sulfate is (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol sulfate (salt) (2:1). A... |
| Active Ingredient | Abacavir sulfate |
| Dosage Form | Solution; Tablet |
| Route | oral; Oral |
| Strength | 300mg; 60mg; 60 mg; 20mg; eq 300mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Hetero Labs Ltd Iii; Mylan Pharms; Aurobindo Pharma; Cipla; Matrix Labs; Apotex; Strides Arcolab |
Abacavir is indicated, in combination with other agents, for treatment of HIV-1 infection. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1
A unique and potentially fatal hypersensitivity reaction occurs in 2% to 5% of patients receiving abacavir. Symptoms typically occur within the first six weeks of therapy and include fever, rash, nausea, malaise, and respiratory complaints, in various combinations. Symptoms initially may be mild but increase in severity with continued administration. Discontinuation of the medication usually resolves all signs and symptoms, but rechallenge may cause rapid onset of severe reactions, hypotension, and death. Once an abacavir hypersensitivity reaction is suspected or confirmed, it is recommended that the patient never by rechallenged with abacavir.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
The major toxicity associated with abacavir therapy is potentially life-threatening hypersensitivity reactions. In clinical studies, hypersensitivity reactions have been reported in approximately 5% of adult and pediatric patients receiving abacavir in conjunction with lamivudine and zidovudine. Fatalities related to hypersensitivity reactions to abacavir have been reported. Manifestations of hypersensitivity usually are apparent within the first 6 weeks of abacavir therapy, but may occur at any time during therapy. Severe hypersensitivity reactions are likely to recur within hours following rechallenge in patients with a prior history of hypersensitivity to the drug, and these reactions may include life-threatening hypotension and death. The most severe hypersensitivity reactions reported to date have been in individuals who were rechallenged with abacavir after a previous hypersensitivity reaction to the drug. There also have been reports of severe or fatal hypersensitivity reactions occurring after abacavir was reintroduced in patients with no identified history of abacavir hypersensitivity or with unrecognized manifestations of hypersensitivity to the drug. Although these patients had discontinued abacavir for reasons unrelated to hypersensitivity (e.g., interruption in drug supply, discontinuance of abacavir during treatment for other medical conditions), some may have had symptoms present before discontinuance of the drug that were consistent with hypersensitivity but were attributed to other medical conditions (e.g., acute onset respiratory disease, gastroenteritis, adverse reactions to other drugs). Most of the hypersensitivity reactions reported following reintroduction of abacavir in these patients were indistinguishable from hypersensitivity reactions associated with abacavir rechallenge (i.e., short time to onset, increased severity of symptoms, poor outcome including death).Hypersensitivity reactions can occur within hours after abacavir is reintroduced; however, in some cases, these reactions occurred days to weeks following reintroduction of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 619
Lactic acidosis and severe hepatomegaly with steatosis (sometimes fatal) have been reported rarely in patients receiving abacavir and also have been reported in patients receiving dideoxynucleoside reverse transcriptase inhibitors. Most reported cases have involved women; obesity and long-term therapy with a nucleoside reverse transcriptase inhibitor also may be risk factors. Increased serum concentrations of Gamma-glutamyltransferase (GGT, GGPT) have been reported in patients receiving abacavir.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 620
Hypersensitivity reactions reported in patients receiving abacavir are characterized by the appearance of manifestations indicating involvement of multiple organ and body systems; these reactions have occurred in association with anaphylaxis, liver failure, renal failure, hypotension, and death. The most frequent manifestations of hypersensitivity reactions to abacavir include fever, rash, fatigue, GI symptoms such as nausea, vomiting, diarrhea, and abdominal pain, and respiratory symptoms such as pharyngitis, dyspnea, and cough. Other signs and symptoms include malaise, lethargy, myalgia, myolysis, headache, arthralgia, edema, paresthesia, lymphadenopathy, and mucous membrane lesions (e.g., conjunctivitis, mouth ulceration). Respiratory symptoms, including cough, dyspnea, and pharyngitis, have been reported in approximately 20% of patients with hypersensitivity reactions to abacavir. Fatalities have occurred in patients who developed hypersensitivity reactions in which the initial presentation included respiratory symptoms; some patients who experienced fatal hypersensitivity reactions were initially diagnosed as having an acute respiratory disease (pneumonia, bronchitis, flu-like illness). Hypersensitivity reactions can occur without rash; if rash occurs, it usually is maculopapular or urticarial, but may be variable in appearance. Laboratory abnormalities reported in patients experiencing a hypersensitivity reaction to abacavir include lymphopenia and increases in serum concentrations of liver enzymes, creatine kinase (CK, creatine phosphokinase, CPK), or creatinine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 619
For more Drug Warnings (Complete) data for ABACAVIR SULFATE (17 total), please visit the HSDB record page.
Ziagen is indicated in antiretroviral combination therapy for the treatment of Human Immunodeficiency Virus (HIV) infection in adults, adolescents and children.
The demonstration of the benefit of Ziagen is mainly based on results of studies performed with a twice daily regimen, in treatment-nave adult patients on combination therapy.
Before initiating treatment with abacavir, screening for carriage of the HLA-B*5701 allele should be performed in any HIV-infected patient, irrespective of racial origin. Abacavir should not be used in patients known to carry the HLA-B*5701 allele.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AF06
Following oral administration of a 600-mg dose of radiolabeled abacavir, 82.2% of the dose is excreted in urine and 16% of the dose is excreted in feces. The 5-carboxylic acid metabolite, 5-glucuronide metabolite, and unchanged abacavir accounted for 30, 36, and 1.2%, respectively, of recovered radioactivity in urine; unidentified minor metabolites accounted for 15% of recovered radioactivity in urine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
It is not known whether abacavir is distributed into human milk; the drug is distributed into milk in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
Abacavir crosses the placenta in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
The oral bioavailability of abacavir is high with or without food; the CSF-to-plasma AUC ratio is approximately 0.3.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
For more Absorption, Distribution and Excretion (Complete) data for ABACAVIR SULFATE (7 total), please visit the HSDB record page.
Abacavir is partially metabolized by alcohol dehydrogenase (to form the 5'-carboxylic acid) and glucuronidation (to form the 5'-glucuronide).
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
The metabolic fate of abacavir has not been fully determined, but the drug is metabolized in the liver. Abacavir is metabolized by alcohol dehydrogenase to form the 5-carboxylic acid and by glucuronyltransferase to form the 5-glucuronide; these metabolites do not appear to have any antiviral activity. Any involvement of cytochrome p450 isoenzymes in the metabolism of abacavir is limited.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
Intracellularly, abacavir is phosphorylated to abacavir monophosphate by adenosine phosphotransferase; abacavir monophosphate is then converted to carbovir monophosphate in a reaction catalyzed by cytosolic enzymes and then to carbovir triphosphate by cellular kinases. Intracellular (host cell) conversion of abacavir to carbovir triphosphate is necessary for the antiviral activity of the drug. The in vitro intracellular half-life of carbovir triphosphate in CD4+ CEM cells is 3.3 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
The in vitro intracellular half-life of carbovir triphosphate /SRP: a metabolite of abacavir sulfate,/ in CD4+ CEM cells is 3.3 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
The plasma elimination half-life of abacavir following a single oral dose (given as abacavir sulfate) is about 1.5 hours. In HIV-infected children 3 months to 13 years of age who received 8 mg/kg of abacavir every 12 hours (given as an oral solution containing abacavir sulfate), steady-state plasma elimination half-life averaged 1.3 hours and was essentially the same as that reported after a single dose. Following oral administration of a single 300-mg dose of abacavir to an individual with renal failure (glomerular filtration rate less than 10 mL/minute) undergoing peritoneal dialysis, the plasma elimination half-life of the drug was 1.33 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 623
Like dideoxynucleoside reverse transcriptase inhibitors (e.g., didanosine, lamivudine, stavudine, zalcitabine, zidovudine), the antiviral activity of abacavir appears to depend on intracellular conversion of the drug to a 5-triphosphate metabolite; thus, carbovir triphosphate (carbocyclic guanosine triphosphate) and not unchanged abacavir appears to be the pharmacologically active form of the drug. Substantial differences exist in the rates at which human cells phosphorylate various nucleoside antiviral agents and in the enzymatic pathways involved.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
Enzymatic conversion of abacavir to carbovir triphosphate appears to be complex and involves certain steps and enzymes that differ from those involved in the enzymatic conversion of dideoxynucleoside reverse transcriptase inhibitors. Abacavir is phosphorylated by adenosine phosphotransferase to abacavir monophosphate, which is converted to carbovir monophosphate by a cytosolic enzyme. Subsequently, carbovir monophosphate is phosphorylated by cellular kinases to carbovir triphosphate. Abacavir is not a substrate for enzymes (i.e., thymidine kinase, deoxycytidine kinase, adenosine kinase, mitochondrial deoxyguanosine kinase) known to phosphorylate other nucleoside analogs. Because phosphorylation of abacavir depends on cellular rather than viral enzymes, conversion of the drug to the active triphosphate derivative occurs in both virus-infected and uninfected cells. Carbovir triphosphate is a structural analog of deoxyguanosine-5-triphosphate (dGTP), the usual substrate for viral RNA-directed DNA polymerase. Although other mechanisms may be involved in the antiretroviral activity of the drug, carbovir triphosphate appears to compete with deoxyguanosine-5-triphosphate for viral RNA-directed DNA polymerase and incorporation into viral DNA. Following incorporation of carbovir triphosphate into the viral DNA chain instead of deoxyguanosine-5-triphosphate, DNA synthesis is prematurely terminated because the absence of the 3-hydroxy group on the drug prevents further 5 to 3 phosphodiester linkages.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621
The complete mechanism(s) of antiviral activity of abacavir has not been fully elucidated. Following conversion to a pharmacologically active metabolite, abacavir apparently inhibits replication of retroviruses, including human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2), by interfering with viral RNA-directed DNA polymerase (reverse transcriptase). The drug, therefore, exerts a virustatic effect against retroviruses by acting as a reverse transcriptase inhibitor.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 621

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
50
PharmaCompass offers a list of Abacavir Sulfate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Abacavir Sulfate manufacturer or Abacavir Sulfate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Abacavir Sulfate manufacturer or Abacavir Sulfate supplier.
PharmaCompass also assists you with knowing the Abacavir Sulfate API Price utilized in the formulation of products. Abacavir Sulfate API Price is not always fixed or binding as the Abacavir Sulfate Price is obtained through a variety of data sources. The Abacavir Sulfate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Abacavir Sulfate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Abacavir Sulfate, including repackagers and relabelers. The FDA regulates Abacavir Sulfate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Abacavir Sulfate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Abacavir Sulfate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Abacavir Sulfate supplier is an individual or a company that provides Abacavir Sulfate active pharmaceutical ingredient (API) or Abacavir Sulfate finished formulations upon request. The Abacavir Sulfate suppliers may include Abacavir Sulfate API manufacturers, exporters, distributors and traders.
click here to find a list of Abacavir Sulfate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Abacavir Sulfate DMF (Drug Master File) is a document detailing the whole manufacturing process of Abacavir Sulfate active pharmaceutical ingredient (API) in detail. Different forms of Abacavir Sulfate DMFs exist exist since differing nations have different regulations, such as Abacavir Sulfate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Abacavir Sulfate DMF submitted to regulatory agencies in the US is known as a USDMF. Abacavir Sulfate USDMF includes data on Abacavir Sulfate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Abacavir Sulfate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Abacavir Sulfate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Abacavir Sulfate Drug Master File in Japan (Abacavir Sulfate JDMF) empowers Abacavir Sulfate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Abacavir Sulfate JDMF during the approval evaluation for pharmaceutical products. At the time of Abacavir Sulfate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Abacavir Sulfate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Abacavir Sulfate Drug Master File in Korea (Abacavir Sulfate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Abacavir Sulfate. The MFDS reviews the Abacavir Sulfate KDMF as part of the drug registration process and uses the information provided in the Abacavir Sulfate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Abacavir Sulfate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Abacavir Sulfate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Abacavir Sulfate suppliers with KDMF on PharmaCompass.
A Abacavir Sulfate CEP of the European Pharmacopoeia monograph is often referred to as a Abacavir Sulfate Certificate of Suitability (COS). The purpose of a Abacavir Sulfate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Abacavir Sulfate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Abacavir Sulfate to their clients by showing that a Abacavir Sulfate CEP has been issued for it. The manufacturer submits a Abacavir Sulfate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Abacavir Sulfate CEP holder for the record. Additionally, the data presented in the Abacavir Sulfate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Abacavir Sulfate DMF.
A Abacavir Sulfate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Abacavir Sulfate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Abacavir Sulfate suppliers with CEP (COS) on PharmaCompass.
A Abacavir Sulfate written confirmation (Abacavir Sulfate WC) is an official document issued by a regulatory agency to a Abacavir Sulfate manufacturer, verifying that the manufacturing facility of a Abacavir Sulfate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Abacavir Sulfate APIs or Abacavir Sulfate finished pharmaceutical products to another nation, regulatory agencies frequently require a Abacavir Sulfate WC (written confirmation) as part of the regulatory process.
click here to find a list of Abacavir Sulfate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Abacavir Sulfate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Abacavir Sulfate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Abacavir Sulfate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Abacavir Sulfate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Abacavir Sulfate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Abacavir Sulfate suppliers with NDC on PharmaCompass.
Abacavir Sulfate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Abacavir Sulfate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Abacavir Sulfate GMP manufacturer or Abacavir Sulfate GMP API supplier for your needs.
A Abacavir Sulfate CoA (Certificate of Analysis) is a formal document that attests to Abacavir Sulfate's compliance with Abacavir Sulfate specifications and serves as a tool for batch-level quality control.
Abacavir Sulfate CoA mostly includes findings from lab analyses of a specific batch. For each Abacavir Sulfate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Abacavir Sulfate may be tested according to a variety of international standards, such as European Pharmacopoeia (Abacavir Sulfate EP), Abacavir Sulfate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Abacavir Sulfate USP).
