
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
DRUG PRODUCT COMPOSITIONS
0
FDF
0
Weekly News Recap #Phispers
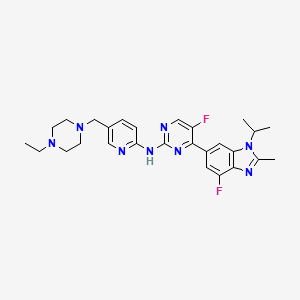

1. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzimidazol-5-yl)pyrimidin-2-yl)amine
2. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzoimidazol-5-yl)pyrimidin-2-yl)amine
3. Abemaciclib Mesylate
4. Ly-2835219
5. Ly2385219
6. Ly2835210
7. Ly2835219
8. Verzenio
1. 1231929-97-7
2. Ly2835219
3. Verzenio
4. Ly2835219 Free Base
5. Ly-2835219
6. Unii-60uab198hk
7. N-(5-((4-ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1h-benzo[d]imidazol-6-yl)pyrimidin-2-amine
8. N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine
9. Abemaciclib (ly2835219)
10. 60uab198hk
11. Ly 2835219
12. Hy-16297a
13. Cs-1230
14. 2-pyrimidinamine, N-(5-((4-ethyl-1-piperazinyl)methyl)-2-pyridinyl)-5-fluoro-4-(4-fluoro-2-methyl-1-(1-methylethyl)-1h-benzimidazol-6-yl)
15. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-1,3-benzodiazol-5-yl)pyrimidin-2-amine
16. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-[4-fluoro-2-methyl-1-(propan-2-yl)-1h-benzimidazol-6-yl]pyrimidin-2-amine
17. Ly2835219 (free Base)
18. Abemaciclib [usan:inn]
19. Abemaciclib,ly2835219
20. Verzenios
21. Rimidin-2-amine
22. Verzenio (tn)
23. 6zv
24. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-[4-fluoro-2-methyl-1-(propan-2-yl)-1h-benzimidazol-6-yl]py Rimidin-2-amine
25. Cdk4/6 Dual Inhibitor
26. Ly2835210
27. Abemaciclib [mi]
28. Abemaciclib [inn]
29. Abemaciclib [jan]
30. Abemaciclib (jan/usan)
31. Abemaciclib [usan]
32. Abemaciclib [who-dd]
33. Gtpl7382
34. Schembl2487229
35. Chembl3301610
36. Abemaciclib [orange Book]
37. Dtxsid20673119
38. Ex-a521
39. Ly 2835219 (free Base)
40. Hms3673i05
41. Bcp13079
42. Ex-a1588
43. Bdbm50110183
44. Mfcd22665744
45. Nsc768073
46. Nsc783671
47. S5716
48. Zinc72318121
49. 1231929-97-7, Verzenio,
50. Akos025404907
51. Ly2835219 Free Base (abemaciclib)
52. Ccg-269750
53. Db12001
54. Nsc-768073
55. Nsc-783671
56. Sb16476
57. Ncgc00351599-02
58. Ncgc00351599-06
59. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzimidazol-5-yl)pyrimidin-2-yl)amine
60. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzoimidazol-5-yl)pyrimidin-2-yl)amine
61. Ac-30666
62. As-10230
63. Da-33422
64. Ly2835219 Ms Salt, Abemaciclib Ms Salt
65. Ft-0700134
66. Ly 2835210
67. A12989
68. D10688
69. J-690083
70. Q23901483
71. [5-(4-ethyl-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzoimidazol-5-yl)-pyrimidin-2-yl]-amine
72. 2-pyrimidinamine, N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1h-benzimidazol-6-yl]-
73. 2-pyrimidinamine,n-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1h-benzimidazol-6-yl]-
74. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-[4-fluoro-2-methyl-1-(propan-2-yl)-1h-benzimidazol-6-yl]py
| Molecular Weight | 506.6 g/mol |
|---|---|
| Molecular Formula | C27H32F2N8 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 506.27179938 g/mol |
| Monoisotopic Mass | 506.27179938 g/mol |
| Topological Polar Surface Area | 75 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 723 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | VERZENIO |
| Active Ingredient | ABEMACICLIB |
| Company | ELI LILLY AND CO (Application Number: N208716. Patent: 7855211) |
* Indicated in combination with fulvestrant for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy. * Inidicated as monotherapy for the treatment of adult patients with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting.
Early Breast Cancer
Verzenios in combination with endocrine therapy is indicated for the adjuvant treatment of adult patients with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative, node positive early breast cancer at high risk of recurrence (see section 5. 1).
In pre or perimenopausal women, aromatase inhibitor endocrine therapy should be combined with a luteinising hormone-releasing hormone (LHRH) agonist.
Advanced or Metastatic Breast Cancer
Verzenios is indicated for the treatment of women with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy.
In pre- or perimenopausal women, the endocrine therapy should be combined with a LHRH agonist.
Treatment of Ewing sarcoma
Treatment of breast cancer
Treatment of high-grade glioma, Treatment of neuroblastoma
In combination with fulvestrant, the progression-free survival for patients with HR-positive, HER2-negative breast cancer was 16.4 months compared to 9.3 months for patients taking a placebo with fulvestrant. As a monotherapy, 19.7% of patients taking abemaciclib achieved complete or partial shrinkage of their tumors for a median 8.6 months after treatment. Abemaciclib induces cell cycle arrest and exerts an antitumor activity in human tumor xenograft models. In patient investigations and a healthy volunteer study, abemaciclib is not shown to induce any clinically significant changes in the QTc interval.
L01EF03
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EF - Cyclin-dependent kinase (cdk) inhibitors
L01EF03 - Abemaciclib
Absorption
The plasma concentration of the drug increases in a dose-proportional manner. Following a single oral dose administration of 200 mg abemaciclib, the mean peak plasma concentration (Cmax) of 158 ng/mL is reached after 6 hours. The median time to reach maximum plasma concentration (Tmax) ranges from 4-6 hours following an oral administration of abemaciclib over a range of 50275 mg, but may range up to 24 hours. The absolute bioavailability of the drug is reported to be 45%.
Route of Elimination
Following a single oral dose of 150mg radiolabeled abemaciclib, approximately 81% of the total dose was recovered in feces while 3% of the dose was detected in urine. The majority of the drug is exceted as metabolites.
Volume of Distribution
The geometric mean systemic volume of distribution is approximately 690.3 L (49% CV).
Clearance
The geometric mean hepatic clearance (CL) of abemaciclib in patients was 26.0 L/h (51% CV).
Abemaciclib mainly undergoes hepatic metabolism mediated by CYP3A4. The major metabolite formed is N-desethylabemaciclib (M2), while other metabolites hydroxyabemaciclib (M20), hydroxy-N-desethylabemaciclib (M18), and an oxidative metabolite (M1) are also formed. M2, M18, and M20 are equipotent to abemaciclib and their AUCs accounted for 25%, 13%, and 26% of the total circulating analytes in plasma, respectively.
The mean plasma elimination half-life for abemaciclib in patients was 18.3 hours (72% CV).
Regulation of cell cycle is crucial in maintaining proper cell growth; dysregulated cell cycle signalling pathway is a key component in inducing hyperproliferation of cells and tumor formation in various cancers. G1 to S phase cell cycle progression, or transition through the G1 restriction point (R), is promoted by the retinoblastoma tumor suppressor protein (Rb)-mediated pathway. Activation of Rb-mediated pathway requires the interaction of Cyclin-dependent kinases (CDK) 4 and 6 with D-type cyclins, which drives the formation of active CDK4/CDK6 and subsequent phosphorylation of Rb. Rb is a tumor suppressant protein that inhibits proliferation through binding to and suppressing the activity of the E2F family of transcription factors. However, phosphorylation of Rb relieves suppression of E2F to allow expression of genes required for passage through the restriction point. This leads to increased expression of downstream signalling molecules and activity of protein kinases that promote the cell cycle progression and initiation of DNA replication. Phosphorylation of Rb and other proteins by CDK4/6 additionally leads to transcription of genes involved in cell cycle-independent activities including signal transduction, DNA repair transcriptional control, and mRNA processing. Abemaciclib selectively inhibits CDK4 and CDK6 with low nanomolar potency, inhibits Rb phosphorylation resulting in a G1 arrest and inhibition of proliferation, and its activity is specific for Rb-proficient cells. Unlike other CDK inhibitors such as [DB09073] and [DB11730], abemaciclib exhibits greater selectivity for CDK4 compared to CDK6.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
14
PharmaCompass offers a list of Abemaciclib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Abemaciclib manufacturer or Abemaciclib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Abemaciclib manufacturer or Abemaciclib supplier.
PharmaCompass also assists you with knowing the Abemaciclib API Price utilized in the formulation of products. Abemaciclib API Price is not always fixed or binding as the Abemaciclib Price is obtained through a variety of data sources. The Abemaciclib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Abemaciclib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Abemaciclib, including repackagers and relabelers. The FDA regulates Abemaciclib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Abemaciclib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Abemaciclib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Abemaciclib supplier is an individual or a company that provides Abemaciclib active pharmaceutical ingredient (API) or Abemaciclib finished formulations upon request. The Abemaciclib suppliers may include Abemaciclib API manufacturers, exporters, distributors and traders.
click here to find a list of Abemaciclib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Abemaciclib DMF (Drug Master File) is a document detailing the whole manufacturing process of Abemaciclib active pharmaceutical ingredient (API) in detail. Different forms of Abemaciclib DMFs exist exist since differing nations have different regulations, such as Abemaciclib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Abemaciclib DMF submitted to regulatory agencies in the US is known as a USDMF. Abemaciclib USDMF includes data on Abemaciclib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Abemaciclib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Abemaciclib suppliers with USDMF on PharmaCompass.
A Abemaciclib written confirmation (Abemaciclib WC) is an official document issued by a regulatory agency to a Abemaciclib manufacturer, verifying that the manufacturing facility of a Abemaciclib active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Abemaciclib APIs or Abemaciclib finished pharmaceutical products to another nation, regulatory agencies frequently require a Abemaciclib WC (written confirmation) as part of the regulatory process.
click here to find a list of Abemaciclib suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Abemaciclib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Abemaciclib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Abemaciclib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Abemaciclib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Abemaciclib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Abemaciclib suppliers with NDC on PharmaCompass.
Abemaciclib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Abemaciclib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Abemaciclib GMP manufacturer or Abemaciclib GMP API supplier for your needs.
A Abemaciclib CoA (Certificate of Analysis) is a formal document that attests to Abemaciclib's compliance with Abemaciclib specifications and serves as a tool for batch-level quality control.
Abemaciclib CoA mostly includes findings from lab analyses of a specific batch. For each Abemaciclib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Abemaciclib may be tested according to a variety of international standards, such as European Pharmacopoeia (Abemaciclib EP), Abemaciclib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Abemaciclib USP).
