
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
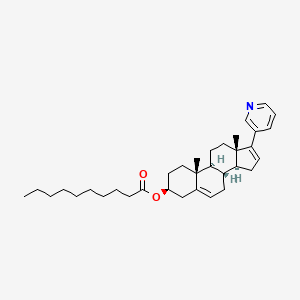

1. Prl-02
2. U6xms339f5
3. 2486052-18-8
4. Unii-u6xms339f5
5. Schembl22414033
6. Ex-a6907
7. Hy-145786
8. Cs-0433104
| Molecular Weight | 503.8 g/mol |
|---|---|
| Molecular Formula | C34H49NO2 |
| XLogP3 | 9.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 11 |
| Exact Mass | 503.376329806 g/mol |
| Monoisotopic Mass | 503.376329806 g/mol |
| Topological Polar Surface Area | 39.2 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 860 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?