


API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
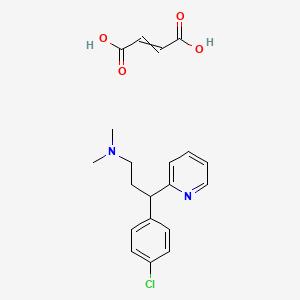

1. 113-92-8
2. Chloropheniramine Maleate
3. R-chlorpheniramine Maleate
4. Chlorphenamine Maleate,(s)
5. Hms3369f09
6. Hms3371m22
7. Hms3373a05
8. Hms3393l14
9. Hms3394e15
10. Hms3655g14
11. Bcp09631
12. Akos030228309
13. Sb19136
14. Db-041191
15. Ft-0623712
16. Ft-0665003
17. Ft-0665004
18. Ft-0665005
19. Z2210694610
| Molecular Weight | 390.9 g/mol |
|---|---|
| Molecular Formula | C20H23ClN2O4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 390.1346349 g/mol |
| Monoisotopic Mass | 390.1346349 g/mol |
| Topological Polar Surface Area | 90.7 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 368 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 1 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | HYDROCODONE BITARTRATE AND CHLORPHENIRAMINE MALEATE |
| Active Ingredient | CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE |
| Company | ACELLA PHARMS LLC (Application Number: A206891) |
| 2 of 6 | |
|---|---|
| Drug Name | HYDROCODONE BITARTRATE, CHLORPHENIRAMINE MALEATE AND PSEUDOEPHEDRINE HYDROCHLORIDE |
| Active Ingredient | CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE; PSEUDOEPHEDRINE HYDROCHLORIDE |
| Company | PADDOCK LLC (Application Number: A204627) |
| 3 of 6 | |
|---|---|
| Drug Name | VITUZ |
| Active Ingredient | CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE |
| Company | CYPRESS PHARM (Application Number: N204307) |
| 4 of 6 | |
|---|---|
| Drug Name | HYDROCODONE BITARTRATE, CHLORPHENIRAMINE MALEATE AND PSEUDOEPHEDRINE HYDROCHLORIDE |
| Active Ingredient | CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE; PSEUDOEPHEDRINE HYDROCHLORIDE |
| Company | BIO-PHARM INC (Application Number: A206660) |
| 5 of 6 | |
|---|---|
| Drug Name | HYDROCODONE BITARTRATE,CHLORPHENIRAMINE MALEATE AND PSEUDOEPHEDRINE HYDROCHLORIDE |
| Active Ingredient | CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE; PSEUDOEPHEDRINE HYDROCHLORIDE |
| Company | MAYNE PHARMA INC (Application Number: A205657) |
| 6 of 6 | |
|---|---|
| Drug Name | ZUTRIPRO |
| Active Ingredient | CHLORPHENIRAMINE MALEATE; HYDROCODONE BITARTRATE; PSEUDOEPHEDRINE HYDROCHLORIDE |
| Company | CYPRESS PHARM (Application Number: N022439) |


