
Synopsis
Synopsis
0
EU WC
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
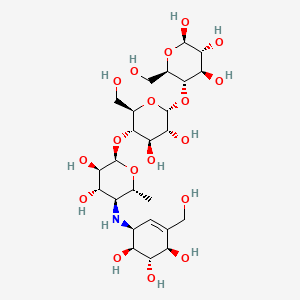

1. Bay G 5421
2. Glucobay
3. Glucor
4. Glumida
5. Prandase
6. Precose
1. Glucobay
2. Precose
3. Prandase
4. Bay-g-5421
5. Bay-g 5421
6. Beta-acarbose
7. 56180-94-0
8. Bay G 5421
9. Chembl1566
10. Alpha-acarbose
11. Glucor
12. Dsstox_cid_26034
13. Dsstox_rid_81300
14. Dsstox_gsid_46034
15. Cas-56180-94-0
16. Arcabose
17. Ascarbose
18. 1agm
19. 1ukt
20. Nsc-758915
21. Ncgc00159353-02
22. Qps
23. Acarbose, >=95%
24. Schembl16848
25. Gtpl6791
26. Chebi:94035
27. Bcpp000442
28. Hms3269l19
29. Hms3413p19
30. Hms3677p19
31. Hms3713b18
32. Tox21_111597
33. Bdbm50333465
34. S1271
35. Zinc85537042
36. Akos024457233
37. Tox21_111597_1
38. Bcp9000224
39. Ccg-220568
40. Ncgc00160515-02
41. Ab01274765-01
42. Ab01274765_02
43. A830944
44. Q338005
45. Brd-k44276885-001-01-7
46. Brd-k44276885-001-07-4
47. Acarbose, European Pharmacopoeia (ep) Reference Standard
48. Acarbose, United States Pharmacopeia (usp) Reference Standard
49. Acarbose For Identification, European Pharmacopoeia (ep) Reference Standard
50. Acarbose, Pharmaceutical Secondary Standard; Certified Reference Material
51. Acarbose For Peak Identification, European Pharmacopoeia (ep) Reference Standard
52. (2r,3r,4r,5r,6r)-5-((2r,3r,4r,5s,6r)-5-((2r,3r,4s,5s,6r)-3,4-dihydroxy-6-methyl-5-((1s,4r,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enylamino)-tetrahydro-2h-pyran-2-yloxy)-3,4-dihydroxy-6-(hydroxymethyl)-tetrahydro-2h-pyran-2-yloxy)-6-(hydroxymethyl)-tetrahydro-2h-pyran-2,3,4-triol
53. (2r,3r,4r,5s,6r)-5-((2r,3r,4r,5s,6r)-5-((2r,3r,4s,5s,6r)-3,4-dihydroxy-6-methyl-5-((1s,4r,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enylamino)-tetrahydro-2h-pyran-2-yloxy)-3,4-dihydroxy-6-(hydroxymethyl)-tetrahydro-2h-pyran-2-yloxy)-6-(hydroxymethyl)-tetrahydro-2h-pyran-2,3,4-triol
54. (2r,3r,4r,5s,6r)-5-((2r,3r,4r,5s,6r)-5-((2r,3r,4s,5s,6r)-3,4-dihydroxy-6-methyl-5-((1s,4s,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enylamino)-tetrahydro-2h-pyran-2-yloxy)-3,4-dihydroxy-6-(hydroxymethyl)-tetrahydro-2h-pyran-2-yloxy)-6-(hydroxymethyl)-tetrahydro-2h-pyran-2,3,4-triol
55. (2r,3r,4r,5s,6r)-5-[(2r,3r,4r,5s,6r)-5-[(2r,3r,4s,5s,6r)-3,4-dihydroxy-6-methyl-5-[[(1s,4r,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)-1-cyclohex-2-enyl]amino]oxan-2-yl]oxy-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-(hydroxymethyl)oxane-2,3,4-triol
56. (3r,4r,5s,6r)-5-((2r,3r,4r,5s,6r)-5-((2r,3r,4s,5s,6r)-3,4-dihydroxy-6-methyl-5-((1s,4r,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-enylamino)-tetrahydro-2h-pyran-2-yloxy)-3,4-dihydroxy-6-(hydroxymethyl)-tetrahydro-2h-pyran-2-yloxy)-6-(hydroxymethyl)-tetrahydro-2h-pyran-2,3,4-triol
57. 4,6-dideoxy-4-([1s]-[1,4,6/5]-4,5,6-trihydroxy-3-hydroxymethyl-2-yclohexenylamino)maltotriose
58. 4-[5-[3,4-dihydroxy-6-methyl-5-[[2,3,4-trihydroxy-5-(hydroxymethyl)cyclohexyl]amino]tetrahydropyran-2-yl]oxy-3,4-dihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-2,3,5,6-tetrahydroxy-hexanal;acarbose
59. 4-o-[4-o-[4-[[(1s,4r,5s,6s)-4,5,6-trihydroxy-3-hydroxymethyl-2-cyclohexene-1-yl]amino]-4,6-dideoxy-alpha-d-glucopyranosyl]-alpha-d-glucopyranosyl]-beta-d-glucopyranose
60. 5-{5-[3,4-dihydroxy-6-methyl-5-(4,5,6-trihydroxy-3-hydroxymethyl-cyclohex-2-enylamino)-tetrahydro-pyran-2-yloxy]-3,4-dihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxy}-6-hydroxymethyl-tetrahydro-pyran-2,3,4-triol
61. D-glucose, O-4,6-dideoxy-4-[[(1s,4r,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-.alpha.-d-glucopyranosyl-(1->4)-o-.alpha.-d-glucopyranosyl-(1->4)-
62. O-4,6-dideoxy-4-[[(1s,4r,5s,6s)-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-?-d-glucopyranosyl-(1-4)-o-?-d-glucopyranosyl-(1-4)-d-glucose
63. Wurcs=2.0/3,3,2/[a2122h-1b_1-5][a2122h-1a_1-5][a2122m-1a_1-5_4*nc^sc^sc^sc^rcco/7=^zc$3/6o/5o/4o]/1-2-3/a4-b1_b4-c1
| Molecular Weight | 645.6 g/mol |
|---|---|
| Molecular Formula | C25H43NO18 |
| XLogP3 | -8.5 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 9 |
| Exact Mass | 645.24801352 g/mol |
| Monoisotopic Mass | 645.24801352 g/mol |
| Topological Polar Surface Area | 321 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 962 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Acarbose |
| PubMed Health | Acarbose (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | Acarbose Tablets are an oral alpha-glucosidase inhibitor for use in the management of type 2 diabetes mellitus. Acarbose is an oligosaccharide which is obtained from fermentation processes of a microorganism, Actinoplanes utahensis, and is chemically... |
| Active Ingredient | Acarbose |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Virtus Pharm; Watson Labs; Strides Pharma; Emcure Pharms; Mylan; Roxane; Impax Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Precose |
| PubMed Health | Acarbose (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | PRECOSE (acarbose tablets) is an oral alpha-glucosidase inhibitor for use in the management of type 2 diabetes mellitus. Acarbose is an oligosaccharide which is obtained from fermentation processes of a microorganism, Actinoplanes utahensis, and is... |
| Active Ingredient | Acarbose |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Acarbose |
| PubMed Health | Acarbose (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | Acarbose Tablets are an oral alpha-glucosidase inhibitor for use in the management of type 2 diabetes mellitus. Acarbose is an oligosaccharide which is obtained from fermentation processes of a microorganism, Actinoplanes utahensis, and is chemically... |
| Active Ingredient | Acarbose |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Virtus Pharm; Watson Labs; Strides Pharma; Emcure Pharms; Mylan; Roxane; Impax Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Precose |
| PubMed Health | Acarbose (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | PRECOSE (acarbose tablets) is an oral alpha-glucosidase inhibitor for use in the management of type 2 diabetes mellitus. Acarbose is an oligosaccharide which is obtained from fermentation processes of a microorganism, Actinoplanes utahensis, and is... |
| Active Ingredient | Acarbose |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
Enzyme Inhibitors; Hypoglycemic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Acarbose tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus./Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
THERAPEUTIC CATEGORY: Antidiabetic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 5
Acarbose is contraindicated in patients with known hypersensitivity to the drug and in patients with diabetic ketoacidosis or cirrhosis. Acarbose is also contraindicated in patients with inflammatory bowel disease, colonic ulceration, partial intestinal obstruction or in patients predisposed to intestinal obstruction. In addition, acarbose is contraindicated in patients who have chronic intestinal diseases associated with marked disorders of digestion or absorption and in patients who have conditions that may deteriorate as a result of increased gas formation in the intestine.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
Because of its mechanism of action, acarbose when administered alone should not cause hypoglycemia in the fasted or postprandial state. Sulfonylurea agents or insulin may cause hypoglycemia. Because acarbose given in combination with a sulfonylurea or insulin will cause a further lowering of blood glucose, it may increase the potential for hypoglycemia. Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, and no increased incidence of hypoglycemia was observed in patients when acarbose was added to metformin therapy. Oral glucose (dextrose), whose absorption is not inhibited by acarbose, should be used instead of sucrose (cane sugar) in the treatment of mild to moderate hypoglycemia. Sucrose, whose hydrolysis to glucose and fructose is inhibited by acarbose, is unsuitable for the rapid correction of hypoglycemia. Severe hypoglycemia may require the use of either intravenous glucose infusion or glucagon injection.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
Gastrointestinal symptoms are the most common reactions to acarbose. ... In a one-year safety study, during which patients kept diaries of gastrointestinal symptoms, abdominal pain and diarrhea tended to return to pretreatment levels over time, and the frequency and intensity of flatulence tended to abate with time. The increased gastrointestinal tract symptoms in patients treated with acarbose are a manifestation of the mechanism of action of acarbose and are related to the presence of undigested carbohydrate in the lower GI tract. If the prescribed diet is not observed, the intestinal side effects may be intensified. If strongly distressing symptoms develop in spite of adherence to the diabetic diet prescribed, the doctor must be consulted and the dose temporarily or permanently reduced.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
In long-term studies (up to 12 months, and including acarbose doses up to 300 mg t.i.d.) conducted in the United States, treatment-emergent elevations of serum transaminases (AST and/or ALT) above the upper limit of normal (ULN), greater than 1.8 times the ULN, and greater than 3 times the ULN occurred in 14%, 6%, and 3%, respectively, of acarbose-treated patients as compared to 7%, 2%, and 1%, respectively, of placebo-treated patients. Although these differences between treatments were statistically significant, these elevations were asymptomatic, reversible, more common in females, and, in general, were not associated with other evidence of liver dysfunction. In addition, these serum transaminase elevations appeared to be dose related. In US studies including acarbose doses up to the maximum approved dose of 100 mg t.i.d., treatment-emergent elevations of AST and/or ALT at any level of severity were similar between acarbose-treated patients and placebo-treated patients (p >/= 0.496).
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
For more Drug Warnings (Complete) data for Acarbose (16 total), please visit the HSDB record page.
Glycoside Hydrolase Inhibitors
Compounds that inhibit or block the activity of GLYCOSIDE HYDROLASES such as ALPHA-AMYLASES and ALPHA-GLUCOSIDASES. (See all compounds classified as Glycoside Hydrolase Inhibitors.)
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BF - Alpha glucosidase inhibitors
A10BF01 - Acarbose
In a study of 6 healthy men, less than 2% of an oral dose of acarbose was absorbed as active drug, while approximately 35% of total radioactivity from a 14C-labeled oral dose was absorbed. An average of 51% of an oral dose was excreted in the feces as unabsorbed drug-related radioactivity within 96 hours of ingestion. Because acarbose acts locally within the gastrointestinal tract, this low systemic bioavailability of parent compound is therapeutically desired.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
Following oral dosing of healthy volunteers with 14C-labeled acarbose, peak plasma concentrations of radioactivity were attained 14-24 hours after dosing, while peak plasma concentrations of active drug were attained at approximately 1 hour. The delayed absorption of acarbose-related radioactivity reflects the absorption of metabolites that may be formed by either intestinal bacteria or intestinal enzymatic hydrolysis.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
Acarbose is metabolized exclusively within the gastrointestinal tract, principally by intestinal bacteria, but also by digestive enzymes. A fraction of these metabolites (approximately 34% of the dose) was absorbed and subsequently excreted in the urine.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
The fraction of acarbose that is absorbed as intact drug is almost completely excreted by the kidneys. When acarbose was given intravenously, 89% of the dose was recovered in the urine as active drug within 48 hours. In contrast, less than 2% of an oral dose was recovered in the urine as active (i.e., parent compound and active metabolite) drug. This is consistent with the low bioavailability of the parent drug.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
For more Absorption, Distribution and Excretion (Complete) data for Acarbose (6 total), please visit the HSDB record page.
Acarbose is metabolized exclusively within the gastrointestinal tract, principally by intestinal bacteria, but also by digestive enzymes. ... At least 13 metabolites have been separated chromatographically from urine specimens. The major metabolites have been identified as 4-methylpyrogallol derivatives (i.e., sulfate, methyl, and glucuronide conjugates). One metabolite (formed by cleavage of a glucose molecule from acarbose) also has alpha-glucosidase inhibitory activity. This metabolite, together with the parent compound, recovered from the urine, accounts for less than 2% of the total administered dose.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
The plasma elimination half-life of acarbose activity is approximately 2 hours in healthy volunteers.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
In contrast to sulfonylureas, acarbose does not enhance insulin secretion. The antihyperglycemic action of acarbose results from a competitive, reversible inhibition of pancreatic alpha-amylase and membrane-bound intestinal alpha-glucoside hydrolase enzymes. Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine, while the membrane-bound intestinal alpha-glucosidases hydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the brush border of the small intestine. In diabetic patients, this enzyme inhibition results in a delayed glucose absorption and a lowering of postprandial hyperglycemia. Because its mechanism of action is different, the effect of acarbose to enhance glycemic control is additive to that of sulfonylureas, insulin or metformin when used in combination. In addition, acarbose diminishes the insulinotropic and weight-increasing effects of sulfonylureas. Acarbose has no inhibitory activity against lactase and consequently would not be expected to induce lactose intolerance.
US Natl Inst Health; DailyMed. Current Medication Information for ACARBOSE tablet (February 2009). Available from, as of October 3, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357
Acarbose represents a pharmacological approach to achieving the metabolic benefits of a slower carbohydrate absorption in diabetes, by acting as a potent, competitive inhibitor of intestinal alpha-glucosidases. Acarbose molecules attach to the carbohydrate binding sites of alpha-glucosidases, with an affinity constant that is much higher than that of the normal substrate. Because of the reversible nature of the inhibitor-enzyme interaction, the conversion of oligosaccharides to monosaccharides is only delayed rather than completely blocked. Acarbose has the structural features of a tetrasaccharide and does not cross the enterocytes after ingestion. Thus, its pharmacokinetic properties are well suited to the pharmacological action directed exclusively towards the intestinal glucosidases. ...
PMID:8906894 Salvatore T, Giugliano D; Clin Pharmacokinet 30 (2): 94-106 (1996)
The aim of the present study was to reveal the possible involvement of thyroid hormones in the antihyperglycaemic and antiperoxidative effects of acarbose. The effects of acarbose on changes in serum concentration of thyroid hormones, insulin and glucose in dexamethasone-induced type 2 diabetic mice were investigated. Simultaneously, changes in lipid peroxidation (LPO), reduced glutathione (GSH) content and the activity of associated endogenous anti-oxidant enzymes, such as superoxide dismuatase (SOD) and catalase (CAT), were investigated in renal and cardiac tissues, which are commonly affected in diabetes mellitus. Although administration of dexamethasone (1.0 mg/kg, i.m., for 22 days) caused hyperglycaemia with a parallel increase in serum insulin and tissue LPO, it decreased thyroid hormone concentrations and the activity of SOD and CAT. When dexamethasone-induced hyperglycemic mice were treated with acarbose (10 mg/kg per day, p.o., for 15 days), levels of thyroid hormones were increased and most of the abnormalities, including serum insulin and glucose levels, tissue LPO, SOD and CAT activity and GSH content, were reversed. These findings suggest the involvement of thyroid hormones in the mode of action of acarbose in amelioration of type 2 diabetes mellitus.
PMID:17042922 Rameshwar J, Anand K; Clin Exp Pharmacol Physiol 33 (11): 1104-6 (2006)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
67
PharmaCompass offers a list of Acarbose API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acarbose manufacturer or Acarbose supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acarbose manufacturer or Acarbose supplier.
PharmaCompass also assists you with knowing the Acarbose API Price utilized in the formulation of products. Acarbose API Price is not always fixed or binding as the Acarbose Price is obtained through a variety of data sources. The Acarbose Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acarbose manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acarbose, including repackagers and relabelers. The FDA regulates Acarbose manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acarbose API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Acarbose manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Acarbose supplier is an individual or a company that provides Acarbose active pharmaceutical ingredient (API) or Acarbose finished formulations upon request. The Acarbose suppliers may include Acarbose API manufacturers, exporters, distributors and traders.
click here to find a list of Acarbose suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acarbose DMF (Drug Master File) is a document detailing the whole manufacturing process of Acarbose active pharmaceutical ingredient (API) in detail. Different forms of Acarbose DMFs exist exist since differing nations have different regulations, such as Acarbose USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acarbose DMF submitted to regulatory agencies in the US is known as a USDMF. Acarbose USDMF includes data on Acarbose's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acarbose USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acarbose suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Acarbose Drug Master File in Japan (Acarbose JDMF) empowers Acarbose API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Acarbose JDMF during the approval evaluation for pharmaceutical products. At the time of Acarbose JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Acarbose suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Acarbose Drug Master File in Korea (Acarbose KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Acarbose. The MFDS reviews the Acarbose KDMF as part of the drug registration process and uses the information provided in the Acarbose KDMF to evaluate the safety and efficacy of the drug.
After submitting a Acarbose KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Acarbose API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Acarbose suppliers with KDMF on PharmaCompass.
A Acarbose CEP of the European Pharmacopoeia monograph is often referred to as a Acarbose Certificate of Suitability (COS). The purpose of a Acarbose CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Acarbose EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Acarbose to their clients by showing that a Acarbose CEP has been issued for it. The manufacturer submits a Acarbose CEP (COS) as part of the market authorization procedure, and it takes on the role of a Acarbose CEP holder for the record. Additionally, the data presented in the Acarbose CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Acarbose DMF.
A Acarbose CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Acarbose CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Acarbose suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Acarbose as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Acarbose API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Acarbose as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Acarbose and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Acarbose NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Acarbose suppliers with NDC on PharmaCompass.
Acarbose Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acarbose GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acarbose GMP manufacturer or Acarbose GMP API supplier for your needs.
A Acarbose CoA (Certificate of Analysis) is a formal document that attests to Acarbose's compliance with Acarbose specifications and serves as a tool for batch-level quality control.
Acarbose CoA mostly includes findings from lab analyses of a specific batch. For each Acarbose CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acarbose may be tested according to a variety of international standards, such as European Pharmacopoeia (Acarbose EP), Acarbose JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acarbose USP).

