
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
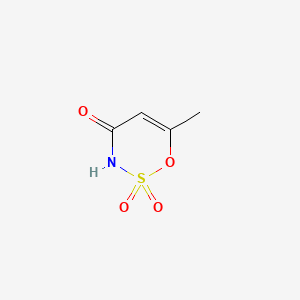

1. Acesulfam-k
2. Acesulfame Calcium
3. Acesulfame K
4. Acesulfame Potassium
5. Acesulfame Sodium
6. Acetosulfam
7. Acetosulfam Potassium
8. Acetosulfam, Potassium Salt
9. Acetosulfam, Sodium Salt
10. Acetosulfame
11. Acetosulfame Calcium
1. 33665-90-6
2. Acetosulfam
3. 6-methyl-1,2,3-oxathiazin-4(3h)-one 2,2-dioxide
4. Acesulfamum
5. Acesulfamo
6. 6-methyl-2,2-dioxooxathiazin-4-one
7. 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one 2,2-dioxide
8. Chebi:83501
9. Acesulfame Potassium
10. Ma3uyz6k1h
11. 6-methyl-1,2,3-oxathiazin-4(3h)-on 2,2-dioxid
12. 1,2,3-oxathiazin-4(3h)-one, 6-methyl-, 2,2-dioxide
13. 3,4-dihydro-6-methyl-1,2,3-oxathiazin-4-one 2,2-dioxide
14. 3,4-dihydro-6-methyl-1,2,3-oxathiazin-4-one-2,2-dioxide
15. Acesulfame-potassium
16. Acetosulfame
17. Acesulfame [ban:inn]
18. Acesulfame [inn:ban]
19. Acesulfamum [inn-latin]
20. Unii-ma3uyz6k1h
21. Acesulfamo [inn-spanish]
22. Hsdb 3914
23. Aud
24. Acesulfame Form Ii
25. Einecs 251-622-6
26. Acesulfame [ii]
27. Acesulfame [mi]
28. Acesulfame [inn]
29. Acesulfame [hsdb]
30. Schembl3551
31. Acesulfame [who-dd]
32. Chembl176687
33. Dtxsid0048006
34. Schembl12166504
35. 6-methyl-2h-1,2lambda~6~,3-oxathiazine-2,2,4(3h)-trione
36. 6-methyl-3,4-dihydro-1,2lambda6,3-oxathiazine-2,2,4-trione
37. Zinc2009976
38. Bbl036697
39. Bdbm50367132
40. Stl559076
41. Akos006273139
42. Vs-13634
43. Ft-0652563
44. E80533
45. Q2823822
46. 6-methyl-1,2,3-oxathiazin-4(3h)-one 2,2-dioxide, 9ci
47. 6-methyl-3,4-dihydro-1,2??,3-oxathiazine-2,2,4-trione
48. [4(3h)-oxo-6-methyl-1,2,3-oxathiazine 2,2-dioxide]-3-ide
49. 6-methyl-3,4-dihydro-1,2$l^{6},3-oxathiazine-2,2,4-trione
50. 6-methyl-3,4-dihydro-1,2,3-oxa-thiazin-4-one 2,2-dioxide
| Molecular Weight | 163.15 g/mol |
|---|---|
| Molecular Formula | C4H5NO4S |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 162.99392881 g/mol |
| Monoisotopic Mass | 162.99392881 g/mol |
| Topological Polar Surface Area | 80.8 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 283 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/MILK/ Nonnutritive sweeteners (NNS), including saccharin, sucralose, aspartame, and acesulfame-potassium, are commonly consumed in the general population, and all except for saccharin are considered safe for use during pregnancy and lactation. Sucralose (Splenda) currently holds the majority of the NNS market share and is often combined with acesulfame-potassium in a wide variety of foods and beverages. To date, saccharin is the only NNS reported to be found in human breast milk after maternal consumption, while there is no apparent information on the other NNS. Breast milk samples were collected from 20 lactating volunteers, irrespective of their habitual NNS intake. Saccharin, sucralose, and acesulfame-potassium were present in 65% of participants' milk samples, whereas aspartame was not detected. These data indicate that NNS are frequently ingested by nursing infants, and thus prospective clinical studies are necessary to determine whether early NNS exposure via breast milk may have clinical implications. /Acesulfame potassium/
PMID:26267522 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5583633 Sylvetsky AC et al; J Toxicol Environ Health A 78 (16): 1029-32 (2015)
Single oral doses of 10 mg (14)C-Acesulfame K/kg bw given to rats and dogs were rapidly absorbed. Maximum blood levels reached were 0.75 plus or minus 0.2 g/mL in rats, 0.5 hr after dosing, and 6.56 plus or minus 2.08 g/mL in dogs, 1-1.5 hr after dosing. In rats, 82-100% of the dose, and in dogs, 85-100% of the dose was excreted in the urine; in both species, 97-100% of the total radioactivity was excreted in feces, and total recovery approximated 100%. Rats given 10 consecutive daily doses of 10 mg/kg orally did not show evidence of accumulation. Three days after dosing, the concn in the organs and plasma was 0.4 nMol/g in liver, and <0.2 nMol/g in other tissues. Seven days after dosing, the concn in dogs was <0.2 nMol/g in all tissues examined. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
After pretreatment for seven days with a diet containing 3% Acesulfame K, male rats were given a dose of 250 mg Acesulfame K containing (14)C-Acesulfame K (9.6 x 108 dpm) by oral gavage. After eight hours the animals were killed and liver and spleen excised; DNA and chromatin protein was isolated from these organs. No radioactivity could be detected on any DNA sample. A low level of activity (8-11 dpm/mg protein) was associated with chromatin protein and this was claimed to be due to non-covalent interactions of unchanged Acesulfame K. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
Single oral doses of approximately 15 mg (14)C-Acesulfame K/kg bw were administered to male and female rats which had been pretreated with unlabeled Acesulfame K at a level of 300 mg/kg diet for 60 days. Control animals without pretreatment were also similarly dosed with (14)C-Acesulfame K. In all animals 95.1-98.2% of the dose was recovered in urine and cage washings and 0.95-2.86% in feces. Total recoveries were 96.3-99.2%. Excretion of radioactivity was rapid and displayed biphasic kinetics; 92.6-96.8% of the dose was excreted in 24 hours. ... No significant differences in route or rate of excretion were observed between sexes nor between controls and animals pretreated with Acesulfame K for 60 days. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
For more Absorption, Distribution and Excretion (Complete) data for Acesulfame (8 total), please visit the HSDB record page.
The metabolism of Acesulfame K was investigated in the urine and feces of rats and dogs which had received single oral doses of 10 mg/kg bw, and in the urine and bile of pigs dosed orally with 5 mg/kg bw. The analytical methods used (thin-layer chromatography, mass spectrometry and isotope dilution) detected only the original substance in these samples. Separation by TLC of urinary extracts from rats used in the above study revealed only one peak which was identical with Acesulfame K. No metabolites were detected in control or Acesulfame K-pretreated animals. Similarly, no metabolites were detected in animals which had been pretreated with 1% Acesulfame K for 7 days. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
The metabolism of Acesulfame K was studied in serum and urine from human volunteers following a single dose of 30 mg/individual. Only the original substance was detected in all samples. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
/After/ single oral doses of approximately 15 mg (14)C-Acesulfame K/kg bw were administered to male and female rats which had been pretreated with unlabeled Acesulfame K at a level of 300 mg/kg diet for 60 days; ... the half-life of the rapid phase was 4-4.5 hours and of the slower phase (accounting for <0.5% of the dose) was 109-257 hours. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
After iv admin of a single dose of 10 mg (14C)-Acesulfame K/kg bw to rats, the radioactivity was excreted quantitatively in urine and the plasma half-life was 0.23 hr. /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
In lactating rats given a single oral dose of (14)C-Acesulfame K of about 10.6 mg/kg bw, ...the biological half-lives were similar in milk (5.6 hr) and blood (4 hr). /Acesulfame K/
WHO Food Additive Series 28: Acesulfame Potassium (1990). Available from, as of October 30, 2017: https://www.inchem.org/documents/jecfa/jecmono/v28je13.htm
The purpose of this work was...the determination of acesulfame-K (AcK) in C57BL mouse plasma and urine. Male and female animals were dosed orally (gavage) and intravenously at 10 mg/kg and then plasma and urine collected at up to 24 hours post dose. Plasma samples were analyzed with an HPLC method... and urine was analyzed with a second HPLC method .... Both methods used saccharin as internal standard with detection by UV absorbance at 230 nm. Following IV administration, plasma AcK concentrations declined rapidly and linearly within 120 min and a second AcK peak was observed at 240 minutes. Half-life was estimated to be 11-15 minutes. Plasma concentrations of AcK reached maximal levels within 45 minutes and rapidly declined following oral doses. Plasma AcK were below limits of detection by 480 minutes post dose. A second peak was also observed following oral administration, suggesting enterohepatic recirculation. Following IV and PO administration, 45% (males) and 70% (females) of the dose was excreted in urine by 24 hr. Oral bioavailability was estimated to be 90-100% based on urinary data.
Lodge J, et al; Toxicol Sci 90 (1-S): 117 (2006)
ABOUT THIS PAGE
91
PharmaCompass offers a list of Acesulfamo API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acesulfamo manufacturer or Acesulfamo supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acesulfamo manufacturer or Acesulfamo supplier.
PharmaCompass also assists you with knowing the Acesulfamo API Price utilized in the formulation of products. Acesulfamo API Price is not always fixed or binding as the Acesulfamo Price is obtained through a variety of data sources. The Acesulfamo Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acesulfamo manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acesulfamo, including repackagers and relabelers. The FDA regulates Acesulfamo manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acesulfamo API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Acesulfamo manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Acesulfamo supplier is an individual or a company that provides Acesulfamo active pharmaceutical ingredient (API) or Acesulfamo finished formulations upon request. The Acesulfamo suppliers may include Acesulfamo API manufacturers, exporters, distributors and traders.
click here to find a list of Acesulfamo suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acesulfamo DMF (Drug Master File) is a document detailing the whole manufacturing process of Acesulfamo active pharmaceutical ingredient (API) in detail. Different forms of Acesulfamo DMFs exist exist since differing nations have different regulations, such as Acesulfamo USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acesulfamo DMF submitted to regulatory agencies in the US is known as a USDMF. Acesulfamo USDMF includes data on Acesulfamo's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acesulfamo USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acesulfamo suppliers with USDMF on PharmaCompass.
Acesulfamo Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acesulfamo GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acesulfamo GMP manufacturer or Acesulfamo GMP API supplier for your needs.
A Acesulfamo CoA (Certificate of Analysis) is a formal document that attests to Acesulfamo's compliance with Acesulfamo specifications and serves as a tool for batch-level quality control.
Acesulfamo CoA mostly includes findings from lab analyses of a specific batch. For each Acesulfamo CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acesulfamo may be tested according to a variety of international standards, such as European Pharmacopoeia (Acesulfamo EP), Acesulfamo JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acesulfamo USP).

