
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
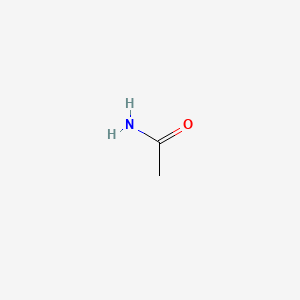

1. Acetamide, Monosodium Salt
1. 60-35-5
2. Ethanamide
3. Acetic Acid Amide
4. Methanecarboxamide
5. Acetimidic Acid
6. Ethanimidic Acid
7. Amide C2
8. Amid Kyseliny Octove
9. Caswell No. 003h
10. Acetimidic Acid (van)
11. Ccris 2
12. Nci-c02108
13. Hsdb 4006
14. Ch3conh2
15. Acetamide, Homopolymer
16. Ai3-02060
17. 8xoe1jso29
18. Chebi:27856
19. Nsc-25945
20. Acetamid
21. 74330-92-0
22. Acetoamide
23. Ethanamid
24. Amid Kyseliny Octove [czech]
25. Einecs 200-473-5
26. Nsc 25945
27. Unii-8xoe1jso29
28. Acetylamine
29. Brn 1071207
30. Essigsaeureamid
31. Imidoacetic Acid
32. N-methylformamde
33. Mfcd00008023
34. Dsstox_cid_5
35. Acetamide, >=98%
36. Acetamide [mi]
37. Acetamide [fhfi]
38. Acetamide [hsdb]
39. Acetamide [iarc]
40. Acetamide, Reagent
41. Lopac-a-0500
42. Bmse000825
43. Bmse000895
44. Ec 200-473-5
45. Acetamide [who-dd]
46. Acetamide, Sublimed, 99%
47. Wln: Zv1
48. Acetic Acid Amide;ethanamide
49. Dsstox_rid_75317
50. Acetamide, ~99% (gc)
51. Dsstox_gsid_20005
52. Lopac0_000003
53. 4-02-00-00399 (beilstein Handbook Reference)
54. Mls002153504
55. Acetamide, Analytical Standard
56. Bidd:er0566
57. Chembl16081
58. Gtpl4661
59. Dtxsid7020005
60. Fema No. 4251
61. Acetamide, Crystalline, >=99%
62. Chebi:49028
63. Acetamide, >=98.0% (gc)
64. Acetamide, >=99.0% (gc)
65. Hms3260a07
66. Acetamide (6ci,7ci,8ci,9ci)
67. Bcp26153
68. Hy-y0946
69. Nsc25945
70. Str01066
71. Zinc8034818
72. Tox21_300776
73. Tox21_500003
74. S6011
75. Stl283915
76. Akos000118788
77. Akos015917387
78. Ccg-204099
79. Db02736
80. Lp00003
81. Sdccgsbi-0049992.p002
82. Cas-60-35-5
83. Benzeneacetic?acid,?
84. A-amino-4-methyl-
85. Ncgc00015030-01
86. Ncgc00015030-02
87. Ncgc00015030-03
88. Ncgc00015030-04
89. Ncgc00015030-05
90. Ncgc00015030-06
91. Ncgc00093530-01
92. Ncgc00093530-02
93. Ncgc00254680-01
94. Ncgc00260688-01
95. Smr000326670
96. A0007
97. Cs-0015934
98. Eu-0100003
99. Ft-0603458
100. Ft-0621721
101. Ft-0621725
102. Ft-0625737
103. A 0500
104. C06244
105. A832706
106. Q421721
107. Sr-01000076247
108. J-523678
109. Sr-01000076247-1
110. Acetamide, Zone-refined, Purified By Sublimation, 99%
111. F1908-0077
112. 02u
| Molecular Weight | 59.07 g/mol |
|---|---|
| Molecular Formula | C2H5NO |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 59.037113783 g/mol |
| Monoisotopic Mass | 59.037113783 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 33 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ /The study objective was/ to observe the effect of fluoroacetamide on cardiomyocytes of rat and the antidotal effect of acetamide. Four groups of SD rats were treated with various dosages of fluoroacetamid (p.o.) and 2 groups of them were treated with acetamide (i.p.). The changes of cardiomyocytes and serum AST, LDH, CK, CK-MB and HBDH were measured at different intervals after poisoning. In the group treated with fluoroacetamid 8 mg/kg. bw, serum AST[(589.58 +/- 821.72) U/L], CK[(916.78 +/- 343.55) U/L], HBDH[(504.47 +/- 148.88) U/L] raised obviously compared with control[(187.70 +/- 46.87), (755.65 +/- 498.90), (347.25 +/- 228.40) U/L respectively] (p<0.01), and the pathological findings such as degeneration, liquefactive necrosis and filtration of inflammatory cells in cardiac muscles were observed 24 hours later, while all the male dead within 3 days. In the group treated with fluoroacetamid 4 mg/kg. bw, serum LDH and HBDH rose significantly compared with control (p<0.01) 5 day later. On the day of 10, myocardial enzymes restored in all experiment groups with some interstitial fibroblastic proliferation. The pathological changes were reduced in the group treated with acetamide synchronously (100 mg/kg. bw). Acute intoxication of fluoroacetamide could damage cardiomyocytes while acetamide could reduce the injury of them, but the injury was reversible. ...
PMID:14694659 Zhu G et al; Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 20 (4): 300-3 (2002)
/EXPL THER/ /The study objective was/ to investigate the effects of acetamide at different doses on the expression of inhibitory amino acids (gamma-aminobutyric acid, GABA) and excitatory amino acid (glutamate, Glu) in the cerebral cortex of rats with acute tetramine (TET) poisoning. Eighty Sprague-Dawley rats (SPF) were randomly divided into five groups, with 16 rats in each group: saline control group, dimethyl sulfoxide (DMSO) control group, TET exposure group, high-dose (2.8 g/kg/d) acetamide treatment group, and super-high-dose (5.6 g/kg/d) acetamide treatment group. Rats in the exposure group and treatment groups were exposed to TET by intragastric administration after fasting, and were then intramuscularly injected with saline or different doses of acetamide in the following 5 days. The cortex of the temporal lobe was collected at 3 hr, 12 hr, 48 hr, or 7 d after treatment. The expression levels of GABA and Glu in the cortex of the temporal lobe were determined by average optical density (OD) values in immunohistochemistry. ... The OD value of GABA in TET exposure group started to increase at 12 hr after treatment, reached the peak at 48 hr, and decreased to the normal level at 7 d. In the high-dose acetamide treatment group, the increase in OD at 12 hr was not so significant as that in the TET exposure group, OD value decreased to the normal level at 48 hr and was lower than that in the exposure group, and the changes were more like those in the control groups. In the super-high-dose acetamide treatment group, OD value began to increase significantly at 3 hr and was significantly higher than that in the TET exposure group (p<0.01), it reached the peak at 12 hr, and was restored to the normal value at 48 hr. ... The OD value of Glu in TET exposure group at 3 hr after treatment was significantly lower than those in the two control groups, it increased gradually from 12 hr to 48 hr, and recovered to the normal level at the 7th d. The changes in the high-dose acetamide treatment group were similar to those in the TET exposure group, but became more like those in the control groups after 48 hr; the OD value in super-high-dose acetamide treatment group was significantly higher than that in the TET exposure group at 3 hr after treatment (p<0.01), while no significant difference was found at 12 hr; it was significantly lower than those of all other groups at 48 hr and 7 d (p<0.01). Treatment with high dose of acetamide has some curative effect on TET poisoning-induced central nervous lesion, while the effect of super-high-dose acetamide on expression of neurotransmitters is too complex to evaluate.
PMID:25169228 Wang X et al; Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 32 (6): 438-41 (2014)
The volume of distribution was about 1 mL/g, total body clearance was 0.27 mL/min and renal clearance was 0.19 mL/min. Approximately 64 72% of (14)C acetamide was excreted in the urine, while only 0.5 0.8% appeared in exhaled air during the first 6 hr after dosing. Thus, approximately 30% of the administered dose was not recovered and it was suggested that metabolized acetamide enters the acetate pool.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V71 1213 (1999)
Less than 0.07% of the recovered urinary radioactivity in rats given 100 or 1000 mg/kg bw (14)C acetamide coeluted upon high performance liquid chromatography with an N-hydroxyacetamide standard and this hydroxamic acid could not be detected after incubation of acetamide with rat liver microsomes and NADPH or in primary cultures of rat hepatocytes. (14)C Acetamide does not bind covalently to proteins in the presence of rat liver microsomes and NADPH or cytosolic fraction, whereas hepatocyte cultures contained non extractable radioactivity. This association was inhibited by cycloheximide to the same extent as (14)C acetate incorporation into cellular proteins.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V71 1213 (1999)
Acetamide ... was found in small amt in human urine as metabolite of metronidazole.
Koch RL et al; Science (Washington, DC 1883-) 211 (4480): 398-400 (1981)
Metabolism of metronidazole to acetamide was apparently mediated by intestinal flora.
Koch RL et al; Biochem Pharmacol 28 (24): 3611-5 (1979)
Acetamide is carcinogenic in rats and mice. To clarify the mechanism of carcinogenesis by acetamide, we investigated DNA damage by andacetamide metabolite, acetohydroxamic acid (AHA), using 32P-5'-end-labeled DNA fragments. AHA treated with amidase induced DNA damage in the presence of Cu(II) and displayed a similar DNA cleavage pattern of hydroxylamine. DNA damage was inhibited by both catalase and bathocuproine, suggesting that H2O2 and Cu(I) are involved. Carboxy-PTIO, a specific scavenger of nitric oxide (NO), partially inhibited DNA damage. The amount of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) by amidase-treated AHA was similar to that by hydroxylamine. ESR spectrometry revealed that amidase-treated AHA as well as hydroxylamine generated NO in the presence of Cu(II). From these results, it has been suggested that AHA might be converted into hydroxylamine by amidase. These results suggest that metal-mediated DNA damage mediated by amidase-catalyzed hydroxylamine generation plays an important role in the carcinogenicity of acetamide.
PMID:15356919 Sakano K et al; Chem Biol Interact 149 (1): 52-9 (2004)
The half life of radioactivity in blood after intravenous dosing of (14)C acetamide to rats averaged 20.6 + or - 0.3 hr after a 10 mg/kg bw dose and 16.1 + or - 1.6 hr after a 50 mg/kg bw dose.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V71 1213 (1999)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
63
PharmaCompass offers a list of Acetamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acetamide manufacturer or Acetamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acetamide manufacturer or Acetamide supplier.
PharmaCompass also assists you with knowing the Acetamide API Price utilized in the formulation of products. Acetamide API Price is not always fixed or binding as the Acetamide Price is obtained through a variety of data sources. The Acetamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acetamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acetamide, including repackagers and relabelers. The FDA regulates Acetamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acetamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Acetamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Acetamide supplier is an individual or a company that provides Acetamide active pharmaceutical ingredient (API) or Acetamide finished formulations upon request. The Acetamide suppliers may include Acetamide API manufacturers, exporters, distributors and traders.
click here to find a list of Acetamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acetamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Acetamide active pharmaceutical ingredient (API) in detail. Different forms of Acetamide DMFs exist exist since differing nations have different regulations, such as Acetamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acetamide DMF submitted to regulatory agencies in the US is known as a USDMF. Acetamide USDMF includes data on Acetamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acetamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acetamide suppliers with USDMF on PharmaCompass.
Acetamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acetamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acetamide GMP manufacturer or Acetamide GMP API supplier for your needs.
A Acetamide CoA (Certificate of Analysis) is a formal document that attests to Acetamide's compliance with Acetamide specifications and serves as a tool for batch-level quality control.
Acetamide CoA mostly includes findings from lab analyses of a specific batch. For each Acetamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acetamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Acetamide EP), Acetamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acetamide USP).

