
Synopsis
Synopsis
0
EU WC
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Actylcystine Gnr
2. Acebraus
3. Acemuc
4. Acetabs
5. Acetylcystein Al
6. Acetylcystein Atid
7. Acetylcystein Heumann
8. Acetylcystein Trom
9. Acetylcystein, Mentopin
10. Acetylcysteine Hydrochloride
11. Acetylcysteine Sodium
12. Acetylcysteine Zinc
13. Acetylcysteine, (d)-isomer
14. Acetylcysteine, (dl)-isomer
15. Acetylcysteine, Monoammonium Salt
16. Acetylcysteine, Monosodium Salt
17. Acetylin
18. Acetyst
19. Acid, Mercapturic
20. Airbron
21. Alveolex
22. Azubronchin
23. Bisolvon Nac
24. Bromuc
25. Broncho Fips
26. Broncho-fips
27. Bronchofips
28. Broncholysin
29. Broncoclar
30. Codotussyl
31. Cystamucil
32. Dampo Mucopect
33. Durabronchal
34. Eurespiran
35. Exomuc
36. Fabrol
37. Fluimucil
38. Fluprowit
39. Frekatuss
40. Genac
41. Hoestil
42. Hustengetrnk, Optipect
43. Hydrochloride, Acetylcysteine
44. Ilube
45. Jenacystein
46. Jenapharm
47. Lantamed
48. Larylin Nac
49. Lindocetyl
50. M Pectil
51. M-pectil
52. Mentopin Acetylcystein
53. Mercapturic Acid
54. Monoammonium Salt Acetylcysteine
55. Monosodium Salt Acetylcysteine
56. Mpectil
57. Muciteran
58. Muco Sanigen
59. Mucomyst
60. Mucopect, Dampo
61. Mucosil
62. Mucosol
63. Mucosolvin
64. N Acetyl L Cysteine
65. N Acetylcysteine
66. N-acetyl-l-cysteine
67. N-acetylcysteine
68. Nac Al
69. Nac Zambon
70. Nac, Bisolvon
71. Optipect Hustengetrnk
72. Sanigen, Muco
73. Siccoral
74. Siran
75. Sodium, Acetylcysteine
76. Solmucol
77. Zambon, Nac
78. Zinc, Acetylcysteine
1. N-acetyl-l-cysteine
2. 616-91-1
3. N-acetylcysteine
4. Mercapturic Acid
5. Acetadote
6. Broncholysin
7. Mucomyst
8. L-acetylcysteine
9. Fluimucetin
10. Fluimucil
11. Parvolex
12. N-acetyl-cysteine
13. Fluprowit
14. Respaire
15. Acetein
16. Airbron
17. Fabrol
18. Mucosil
19. Flumucetin
20. Mucosolvin
21. Brunac
22. Fluimicil Infantil
23. N-acetyl Cysteine
24. Acetilcisteina
25. Acetylcysteinum
26. Lysomucil
27. Mucofilin
28. Syntemucol
29. Acetyl Cysteine
30. Exomuc
31. Inspir
32. Tixair
33. Ac-cys-oh
34. N-acetyl-3-mercaptoalanine
35. Mucolyticum Lappe
36. Mucolytikum Lappe
37. (r)-2-acetamido-3-mercaptopropanoic Acid
38. Acetyl-l-cysteine
39. Mucolyticum
40. Cetylev
41. Cysteine, N-acetyl-, L-
42. N-acetyl-l-(+)-cysteine
43. Neo-fluimucil
44. Nac-tb
45. L-cysteine, N-acetyl-
46. (2r)-2-acetamido-3-sulfanylpropanoic Acid
47. Component Of Naxid
48. Mercapturic Acid, (r)-
49. Cysteine, N-acetyl-
50. (r)-mercapturic Acid
51. Fluatox
52. Fluimicil
53. Mucolator
54. Mucret
55. Oristar Nalc
56. (2r)-2-acetamido-3-sulfanyl-propanoic Acid
57. L-alpha-acetamido-beta-mercaptopropionic Acid
58. N-acetyi-l-cysteine
59. Nsc 111180
60. Mucolyticum-lappe
61. N-acetyl-l-cysteine Hydrochloride
62. N-acetyl-(r)-cysteine
63. Wyq7n0bpyc
64. Nsc-111180
65. Rk-0202
66. Mls000028419
67. Chebi:28939
68. Ncgc00022304-05
69. Lnac
70. Smr000058377
71. Dsstox_cid_21
72. (r)-2-acetylamino-3-mercaptopropanoic Acid
73. (2r)-2-acetylamino-3-sulfanylpropanoic Acid
74. Mfcd00004880
75. Dsstox_rid_75324
76. Dsstox_gsid_20021
77. Flumil
78. Ilube
79. N-acetylcystein
80. Muco Sanigen
81. Mucosil-10
82. Mucosil-20
83. Acetylcysteinum [inn-latin]
84. Cas-616-91-1
85. Acetilcisteina [inn-spanish]
86. Ccris 3764
87. Hsdb 3003
88. Sr-01000075439
89. Unii-wyq7n0bpyc
90. Einecs 210-498-3
91. Mucocedyl
92. Accys
93. Nsc111180
94. N-acetyl-l-cys
95. Sodium 2-acetamido-3-mercaptopropionate
96. Sc2
97. Ilube (eye Drops)
98. N-a-c Sustain
99. N-acetyl-l-cystein
100. Naxid (salt/mix)
101. N-acety-l-cysteine
102. (r)-n-acetylcysteine
103. Acetyl Cysteine,(s)
104. Acetylcysteine [usan:usp:inn:ban:jan]
105. Opera_id_452
106. Mucomyst (tn)
107. Acetylcysteine Ph. Eur.
108. Spectrum2_000086
109. Spectrum3_000287
110. Spectrum4_000137
111. Spectrum5_000764
112. Chembl600
113. Nac & Tnf
114. Acetylcysteine [ii]
115. Acetylcysteine [mi]
116. Schembl5292
117. Acetylcysteine [inn]
118. Acetylcysteine [jan]
119. Lopac0_000081
120. Acetylcysteine [hsdb]
121. Acetylcysteine [usan]
122. Bspbio_001794
123. Kbiogr_000554
124. Mls001076125
125. Mls006011563
126. Acetylcysteine [vandf]
127. Spectrum1500105
128. Spbio_000012
129. Acetyl Cysteine [inci]
130. Acetylcysteine [mart.]
131. Acetylcysteine [usp-rs]
132. Acetylcysteine [who-dd]
133. Acetylcysteine(n-acetylcysteine)
134. Dtxsid5020021
135. Gtpl10945
136. Kbio3_001294
137. N-acetyl-l-cysteine, Usp Grade
138. Acetylcysteine (jp17/usp/inn)
139. Hms1920a11
140. Hms2091g11
141. Hms2234j22
142. Hms3260a04
143. Hms3655g11
144. Hms3715d03
145. Hms3884e04
146. Hy-b0215
147. Zinc3589203
148. 2-acetylamino-3-mercapto-propionate
149. Acetylcysteine [orange Book]
150. Tox21_110877
151. Tox21_201078
152. Tox21_500081
153. Acetylcysteine (n-acetyl-l-cysteine)
154. Acetylcysteine [ep Monograph]
155. Bdbm50420190
156. Ccg-38902
157. S1623
158. Acetylcysteine [usp Monograph]
159. Akos015841009
160. Tox21_110877_1
161. Ccg-204176
162. Db06151
163. Gs-3121
164. Lp00081
165. Sdccgsbi-0050069.p002
166. (r)-2-acetamido-3-mercaptopropanoicacid
167. Ncgc00015086-04
168. Ncgc00022304-03
169. Ncgc00022304-04
170. Ncgc00022304-06
171. Ncgc00022304-07
172. Ncgc00022304-08
173. Ncgc00022304-17
174. Ncgc00022304-23
175. Ncgc00258631-01
176. Ncgc00260766-01
177. Ac-16071
178. Ac-24117
179. Bp-12854
180. Sbi-0051272.p003
181. Db-038288
182. A0905
183. Am20100502
184. Eu-0100081
185. Sw199597-2
186. (2r)-2-acetylamino-3-mercapto-propionic Acid
187. En300-72028
188. 16a911
189. A 7250
190. A-1100
191. C06809
192. D00221
193. L-cysteine, N-acetyl- & Tumor Necrosis Factor
194. N-acetyl-l-cysteine, Bioxtra, >=99% (tlc)
195. Ab00051908_02
196. Ab00382974-12
197. Ab00382974_13
198. L-.alpha.-acetamido-.beta.-mercaptopropionic Acid
199. Q375613
200. J-507685
201. N-acetyl-l-cysteine & Tumor Necrosis Factor (tnf)
202. N-acetyl-l-cysteine 100 Microg/ml In Acetonitrile
203. Sr-01000075439-1
204. Sr-01000075439-3
205. Sr-01000075439-5
206. Brd-k59058747-001-20-9
207. N-acetyl-l-cysteine, Cell Culture Tested, Bioreagent
208. N-acetyl-l-cysteine, Vetec(tm) Reagent Grade, 98%
209. F1905-7178
210. Cabc898a-e48b-4e13-9f72-98d0609a1854
211. N-acetyl-l-cysteine, Saj Special Grade, 98.0-102.0%
212. N-acetyl-l-cysteine, Sigma Grade, >=99% (tlc), Powder
213. Acetylcysteine, British Pharmacopoeia (bp) Reference Standard
214. Acetylcysteine, European Pharmacopoeia (ep) Reference Standard
215. Acetylcysteine, United States Pharmacopeia (usp) Reference Standard
216. N-acetyl-l-cysteine, Pharmaceutical Secondary Standard; Certified Reference Material
217. N-acetyl-l-cysteine, Pharmagrade, Ajinomoto, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
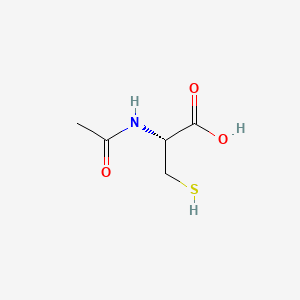
| Molecular Weight | 163.20 g/mol |
|---|---|
| Molecular Formula | C5H9NO3S |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 163.03031432 g/mol |
| Monoisotopic Mass | 163.03031432 g/mol |
| Topological Polar Surface Area | 67.4 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 148 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Acetadote |
| PubMed Health | Acetylcysteine (Injection) |
| Drug Classes | Acetaminophen Antidote |
| Drug Label | Acetylcysteine injection is an intravenous antidote for the treatment of acetaminophen overdose. Acetylcysteine is the nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-acetyl-L-cysteine). The compou... |
| Active Ingredient | Acetylcysteine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 6gm/30ml (200mg/ml) |
| Market Status | Prescription |
| Company | Cumberland Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Acetylcysteine |
| PubMed Health | Acetylcysteine |
| Drug Classes | Acetaminophen Antidote, Amino Acid Supplement, Diagnostic Agent, Bronchial, Mucolytic |
| Drug Label | Acetylcysteine injection is an intravenous antidote for the treatment of acetaminophen overdose. Acetylcysteine is the nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-acetyl-L-cysteine). The compou... |
| Active Ingredient | Acetylcysteine |
| Dosage Form | Injectable; Solution |
| Route | Intravenous; Inhalation, oral |
| Strength | 6gm/30ml (200mg/ml); 10%; 20% |
| Market Status | Prescription |
| Company | Hospira; Luitpold; Innopharma Licensing |
| 3 of 4 | |
|---|---|
| Drug Name | Acetadote |
| PubMed Health | Acetylcysteine (Injection) |
| Drug Classes | Acetaminophen Antidote |
| Drug Label | Acetylcysteine injection is an intravenous antidote for the treatment of acetaminophen overdose. Acetylcysteine is the nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-acetyl-L-cysteine). The compou... |
| Active Ingredient | Acetylcysteine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 6gm/30ml (200mg/ml) |
| Market Status | Prescription |
| Company | Cumberland Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Acetylcysteine |
| PubMed Health | Acetylcysteine |
| Drug Classes | Acetaminophen Antidote, Amino Acid Supplement, Diagnostic Agent, Bronchial, Mucolytic |
| Drug Label | Acetylcysteine injection is an intravenous antidote for the treatment of acetaminophen overdose. Acetylcysteine is the nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-acetyl-L-cysteine). The compou... |
| Active Ingredient | Acetylcysteine |
| Dosage Form | Injectable; Solution |
| Route | Intravenous; Inhalation, oral |
| Strength | 6gm/30ml (200mg/ml); 10%; 20% |
| Market Status | Prescription |
| Company | Hospira; Luitpold; Innopharma Licensing |
Antiviral Agents; Expectorants; Free Radical Scavengers
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... 113 patients entered into the study were reported to be pregnant at the time of /acetaminophen/ overdose. Follow up including appropriate laboratory and pregnancy data outcome data, was available in 60 cases. Of these, 19 overdosed during the first trimester, 22 during the second trimester and 19 during the third trimester of pregnancy. Of the 24 patients with acetaminophen levels above the acetaminophen overdose nomogram line, 10 were treated with N-acetylcysteine within 10 hr postingestion; eight delivered normal infants, two had elective abortions. Of ten patients treated with N-acetylcysteine 10-16 hr postingestion, five delivered viable infants, two had elective abortions, and three had spontaneous abortions. Of four women treated with N-acetylcysteine 16-24 hr postingestion, one mother died, and there was one spontaneous abortion, one stillbirth, one elective abortion, and one delivery. ...
PMID:2748061 Riggs BS et al; Obstet Gynecol 74 (2): 247-53 (1989)
Acetylcysteine is indicated in the treatment of acetaminophen overdose to protect against hepatotoxicity . /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.
Acetylcysteine is used in current medical practice in conjunction with chest physiotherapy as mucolytic in patients who have viscid or thickened airway mucus. When administered via direct instillation, it is used to loosen impacted mucus plugs during bronchoscopy. Acetylcysteine can irritate the airways and induce bronchospasm when given by inhalation; therefore, it should be administered simultaneously with or following administration of an inhaled beta-adrenergic bronchodilator. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 19
To evaluate the effectiveness and safety of N-acetylcysteine (NAC) in treating chronic hepatitis B patients, 144 patients with chronic hepatitis B (total bilirubin, TBil>170 mmol/L) from several centers were chosen for a randomized and double blind clinical trial. The patients were divided into a NAC group and a placebo group and all of them were treated with an injection containing the same standardized therapeutic drugs. A daily dose of 8 microgram NAC was added to the injection of the NAC group. The trial lasted 45 days. Hepatic function and other biochemistry parameters were checked at the experimental day 0 and days 15, 30, 45. Each group consisted of 72 patients of similar demology and disease characteristics. During the trial, 28 cases of the 144 patients dropped out. In the NAC group, at day 0 and day 30, the TBil were401.7 vs. 149.2 and 160.1+/-160.6. In the placebo group, the TBil on the corresponding days were 384.1+/-134.0 and 216.3+/-199.9. Its decrease in the NAC group was 62% and 42% in the placebo group. At day 0 and day 45 of treatment, the effective PTa increase rate was 72% in the NAC group and 54% in the placebo group. The total effective rate (TBil + PTa) was 90% in the NAC group and 69% in the placebo group. The parameters of the two groups showed a remarkable difference. The rate of side effects was 14% in the NAC and 5% in the placebo groups. NAC can decrease the level of serum TBil, increase the PTa and reduce the time of hospitalization. NAC showed no serious adverse effects during the period of our treatment. We find that NCA is effective and secure in treating chronic hepatitis B patients.
PMID:15670485 Shi XF et al; Zhonghua Gan Zang Bing Za Zhi 13 (1): 20-3 (2005)
... /Acetylcysteine/ should be used during pregnancy only when clearly needed. ... Since it is not known if acetylcysteine is distributed into human milk, the drug should be used with caution in nursing women.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3565
Anaphylactoid reactions (i.e., acute hypersensitivity reactions such as rash, hypotension, wheezing, and/or dyspnea) have been reported in patients receiving IV acetylcysteine for the treatment of acetaminophen overdosage; in some cases, the anaphylactoid reactions were serious, including death in a patient with asthma. Rash, urticaria, and pruritus are the most frequently reported adverse reactions in patients receiving IV acetylcysteine. Acute flushing and erythema also have occurred; these reactions generally occur 30-60 minutes after initiating the infusion and resolve despite infusion of the drug. Reactions to acetylcysteine that involve manifestations other than flushing and erythema should be considered anaphylactoid reactions and treated as such.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3564
Chest tightness and bronchoconstriction have been reported with acetylcysteine. Clinically overt acetylcysteine-induced bronchospasm occurs rarely and unpredictably, even in patients with asthmatic bronchitis or bronchitis complicating bronchial asthma. Occasionally, patients receiving oral inhalation of acetylcysteine develop increased airway obstruction of varying and unpredictable severity. Patients who have had such reactions to previous therapy with acetylcysteine may not react during subsequent therapy with the drug, and patients who have had inhalation treatments with acetylcysteine without incident may react to subsequent therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3564
Nausea, vomiting, and other GI symptoms may occur following oral administration of acetylcysteine in the treatment of acetaminophen overdosage. The drug may also aggravate vomiting associated with acetaminophen overdosage. Administration of dilute acetylcysteine solutions may minimize the tendency of the drug to aggravate vomiting.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3564
For more Drug Warnings (Complete) data for N-ACETYLCYSTEINE (15 total), please visit the HSDB record page.
2. 2= Slightly toxic. Probable oral lethal dose (human) 5-15 g/kg; for 70 kg person (150 lb) between 1 pint and 1 quart.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-380
Acetylcysteine is indicated for mucolytic therapy and in the management of [acetaminophen] overdose.
FDA Label
Acetylcysteine is indicated for mucolytic therapy and in the management of acetaminophen overdose. It has a short duration of action as it is given every 1-8 hours depending on route of administration, and has a wide therapeutic window. Patients should be counselled regarding diluting oral solutions in cola for taste masking, the risk of hypersensitivity, and the risk of upper gastrointestinal hemorrhage.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Expectorants
Agents that increase mucous excretion. Mucolytic agents, that is drugs that liquefy mucous secretions, are also included here. (See all compounds classified as Expectorants.)
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
R05CB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R05 - Cough and cold preparations
R05C - Expectorants, excl. combinations with cough suppressants
R05CB - Mucolytics
R05CB01 - Acetylcysteine
S - Sensory organs
S01 - Ophthalmologicals
S01X - Other ophthalmologicals
S01XA - Other ophthalmologicals
S01XA08 - Acetylcysteine
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB23 - Acetylcysteine
Absorption
An 11 g dose in the form of an effervescent tablet for solution reaches a mean Cmax of 26.5 g/mL, with a Tmax of 2 hours, and an AUC of 186 g\*h/mL.
Route of Elimination
An oral dose of radiolabelled acetylcysteine is 13-38% recovered in the urine in the first 24 hours, while 3% is recovered in the feces.
Volume of Distribution
The volume of distribution of acetylcysteine is 0.47 L/kg.
Clearance
Acetylcysteine has a mean clearance of 0.11 L/hr/kg.
Following oral administration (e.g., when used as an antidote for acetaminophen overdosage), acetylcysteine is absorbed from the GI tract.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3565
Oral acetylcysteine is rapidly absorbed, but the bioavailability is low (10-30%) due to significant first-pass metabolism. Intact acetylcysteine has a relatively small volume of distribution (0.5 L/kg). Serum concentrations after intravenous administration of an initial loading dose of 150 mg/kg over 15 minutes are about 500 mg/L. A steady state plasma concentration of 35 mg/L (10-90 mg/L) was reached in about 12 hours following the loading dose with a continuous infusion of 50 mg/kg over 4 hours and 100 mg/kg over the next 16 hours.
Goldfrank, L.R., Flomenbaum, N.E., Lewin, N.A., Weisman, R.S., Howland, M.A., Hoffman, R.S., Goldfrank's Toxicologic Emergencies 6th Ed. (1998)., McGraw-Hill, New York, N.Y., p. 566
Acetylcysteine can be deacetylated by aminoacylase 1 or other undefined deacetylases before undergoing the normal metabolism of cysteine.
Following oral inhalation or intratracheal instillation, most of the administered drug appears to participate in the sulfhydryl-disulfide reaction; the remainder is absorbed from the pulmonary epithelium, deacetylated by the liver to cysteine, and subsequently metabolized.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3565
Acetylcysteine undergoes rapid deacetylation in vivo to yield cysteine or oxidation to yield diacetylcystine.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 19
The mean terminal half life of acetylcysteine in adults is 5.6 hours and in pre-term neonates is 11 hours.
Following IV administration of acetylcysteine, mean elimination half lives of 5.6 and 11 hours have been reported in adults and in neonates, respectively. The mean elimination half life was increased by 80% in patients with severe liver damage (i.e., alcoholic cirrhosis (Child-Pugh score of 7-13) or primary and/or secondary biliary cirrhosis (Child-Pugh score of 5-11)).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3565
A number of possible mechanisms for the mucolytic activity of acetylcysteine have been proposed. Acetylcysteine's sulfhydryl groups may hydrolize disulfide bonds within mucin, breaking down the oligomers, and making the mucin less viscous. Acetylcysteine has also been shown to reduce mucin secretion in rat models. It is an antioxidant in its own right but is also deacetylated to cysteine, which participates in the synthesis of the antioxidant glutathione. The antioxidant activity may also alter intracellular redox reactions, decreasing phosphorylation of EGFR and MAPK, which decrease transcription of the gene MUC5AC which produces mucin. In the case of acetaminophen overdoses, a portion of the drug is metabolized by CYP2E1 to form the potentially toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI). The amount of NAPQI produced in an overdose saturates and depletes glutathione stores. The free NAPQI promiscuously binds to proteins in hepatocytes, leading to cellular necrosis. Acetylcysteine can directly conjugate NAPQI or provide cysteine for glutathione production and NAPQI conjugation.
Acetylcysteine exerts its mucolytic action through its free sulfhydryl group, which opens the disulfide bonds and lower the viscosity of the mucus. This action increases with increasing pH and is most significant at pH 7 to 9. The mucolytic action of acetylcysteine is not affected by the presence of DNA.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 19
Acetylcysteine may protect against acetaminophen overdose-induced hepatotoxicity by maintaining or restoring hepatic concentrations of glutathione. Glutathione is required to inactivate an intermediate metabolite of acetaminophen that is thought to be hepatotoxic. In acetaminophen overdose, excessive quantities of this metabolite are formed because the primary metabolic (glucuronide and sulfate conjugation) pathways become saturated. Acetylcysteine may act by reducing the metabolite to the parent compound and/or by providing sulfhydryl for conjugation of the metabolite. Experimental evidence also suggests that a sulfhydryl-containing compound such as acetylcysteine may directly inactivate the metabolite.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
73
PharmaCompass offers a list of Acetylcysteine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acetylcysteine manufacturer or Acetylcysteine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acetylcysteine manufacturer or Acetylcysteine supplier.
PharmaCompass also assists you with knowing the Acetylcysteine API Price utilized in the formulation of products. Acetylcysteine API Price is not always fixed or binding as the Acetylcysteine Price is obtained through a variety of data sources. The Acetylcysteine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acetylcysteine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acetylcysteine, including repackagers and relabelers. The FDA regulates Acetylcysteine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acetylcysteine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Acetylcysteine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Acetylcysteine supplier is an individual or a company that provides Acetylcysteine active pharmaceutical ingredient (API) or Acetylcysteine finished formulations upon request. The Acetylcysteine suppliers may include Acetylcysteine API manufacturers, exporters, distributors and traders.
click here to find a list of Acetylcysteine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acetylcysteine DMF (Drug Master File) is a document detailing the whole manufacturing process of Acetylcysteine active pharmaceutical ingredient (API) in detail. Different forms of Acetylcysteine DMFs exist exist since differing nations have different regulations, such as Acetylcysteine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acetylcysteine DMF submitted to regulatory agencies in the US is known as a USDMF. Acetylcysteine USDMF includes data on Acetylcysteine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acetylcysteine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acetylcysteine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Acetylcysteine Drug Master File in Japan (Acetylcysteine JDMF) empowers Acetylcysteine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Acetylcysteine JDMF during the approval evaluation for pharmaceutical products. At the time of Acetylcysteine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Acetylcysteine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Acetylcysteine Drug Master File in Korea (Acetylcysteine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Acetylcysteine. The MFDS reviews the Acetylcysteine KDMF as part of the drug registration process and uses the information provided in the Acetylcysteine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Acetylcysteine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Acetylcysteine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Acetylcysteine suppliers with KDMF on PharmaCompass.
A Acetylcysteine CEP of the European Pharmacopoeia monograph is often referred to as a Acetylcysteine Certificate of Suitability (COS). The purpose of a Acetylcysteine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Acetylcysteine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Acetylcysteine to their clients by showing that a Acetylcysteine CEP has been issued for it. The manufacturer submits a Acetylcysteine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Acetylcysteine CEP holder for the record. Additionally, the data presented in the Acetylcysteine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Acetylcysteine DMF.
A Acetylcysteine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Acetylcysteine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Acetylcysteine suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Acetylcysteine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Acetylcysteine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Acetylcysteine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Acetylcysteine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Acetylcysteine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Acetylcysteine suppliers with NDC on PharmaCompass.
Acetylcysteine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acetylcysteine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acetylcysteine GMP manufacturer or Acetylcysteine GMP API supplier for your needs.
A Acetylcysteine CoA (Certificate of Analysis) is a formal document that attests to Acetylcysteine's compliance with Acetylcysteine specifications and serves as a tool for batch-level quality control.
Acetylcysteine CoA mostly includes findings from lab analyses of a specific batch. For each Acetylcysteine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acetylcysteine may be tested according to a variety of international standards, such as European Pharmacopoeia (Acetylcysteine EP), Acetylcysteine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acetylcysteine USP).
