
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aarane
2. Acid, Cromoglicic
3. Acid, Cromoglycic
4. Bicromat Spray
5. Cromoglicic Acid
6. Cromoglycate
7. Cromoglycate, Disodium
8. Cromoglycate, Sodium
9. Cromoglycic Acid
10. Cromolyn Sodium
11. Disodium Cromoglycate
12. Fpl 670
13. Fpl-670
14. Fpl670
15. Intal
16. Lomudal
17. Nalcrom
18. Nasalcrom
19. Opticrom
20. Sodium Cromoglycate
21. Vicrom
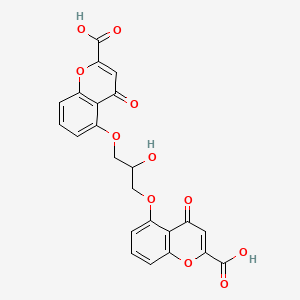
1. Cromoglicic Acid
2. 16110-51-3
3. Cromoglycic Acid
4. Cromoglycate
5. Cromoglicate
6. Acidum Cromoglicicum
7. Intal
8. 5,5'-((2-hydroxypropane-1,3-diyl)bis(oxy))bis(4-oxo-4h-chromene-2-carboxylic Acid)
9. Acido Cromoglicico
10. Acide Cromoglicique
11. Cromo-comod
12. Aarane
13. Acide Cromoglicique [inn-french]
14. Acido Cromoglicico [inn-spanish]
15. Acidum Cromoglicicum [inn-latin]
16. 5-[3-(2-carboxy-4-oxochromen-5-yl)oxy-2-hydroxypropoxy]-4-oxochromene-2-carboxylic Acid
17. Y0tk0fs77w
18. Chebi:59773
19. R03bc01
20. Aararre
21. Frenasma
22. Lomudal
23. Lomudas
24. Lomusol
25. Nalcrom
26. Nebulasma
27. Rynacrom
28. Nasmil
29. 1,3-bis(2-carboxychromon-5-yloxy)-2-hydroxypropane
30. Cromo Asma Aerosol
31. Cromoglicic Acid (inn)
32. Dinatrium Cromoglicicum
33. 5,5'-((2-hydroxypropane-1,3-diyl)bis(oxy))-bis(4-oxo-4h-chromene-2-carboxylic Acid)
34. Cromoglicic Acid [inn]
35. 1,3-di(2-carboxy-4-oxochromen-5-yloxy)propan-2-ol
36. 4h-1-benzopyran-2-carboxylic Acid, 5,5'-((2-hydroxy-1,3-propanediyl)bis(oxy))bis(4-oxo-
37. 4h-1-benzopyran-2-carboxylic Acid, 5,5'-[(2-hydroxy-1,3-propanediyl)bis(oxy)]bis[4-oxo-
38. 5,5'-[(2-hydroxypropane-1,3-diyl)bis(oxy)]bis(4-oxo-4h-chromene-2-carboxylic Acid)
39. Fpl 670
40. 5-[3-(2-carboxy-4-oxo-4h-5-chromenyloxy)-2-hydroxypropoxy]-4-oxo-4h-2-chromenecarboxylic Acid
41. Sodium Chromoglycate
42. 5,5'-(2-hydroxytrimethylenedioxy)bis(4-oxochromene-2-carboxylic Acid)
43. Nsc109500
44. Ncgc00021142-02
45. Unii-y0tk0fs77w
46. Chromoglycate
47. Chromolyn
48. Cromoglicic Acid [inn:ban]
49. Cromoglicic-acid
50. Hsdb 3308
51. 5-(3-((2-carboxy-4-oxo-4h-chromen-5-yl)oxy)-2-hydroxypropoxy)-4-oxo-4h-chromene-2-carboxylic Acid
52. 5-{3-[(2-carboxy-4-oxo-4h-chromen-5-yl)oxy]-2-hydroxypropoxy}-4-oxo-4h-chromene-2-carboxylic Acid
53. Einecs 240-279-8
54. Cromo-comod (tn)
55. Spectrum_000852
56. Cromolyn [hsdb]
57. Cromolyn [mi]
58. Cromolyn [vandf]
59. Cromolyn;cromoglicic Acid
60. Prestwick0_000812
61. Prestwick1_000812
62. Prestwick2_000812
63. Prestwick3_000812
64. Spectrum2_001137
65. Spectrum3_000367
66. Spectrum4_000302
67. Spectrum5_000791
68. Schembl3865
69. Oprea1_077850
70. Bspbio_000703
71. Bspbio_002093
72. Kbiogr_000884
73. Kbioss_001332
74. Bidd:gt0397
75. Divk1c_000857
76. Spbio_001033
77. Spbio_002624
78. Bpbio1_000775
79. Chembl428880
80. Gtpl7608
81. Dtxsid4022860
82. Kbio1_000857
83. Kbio2_001332
84. Kbio2_003900
85. Kbio2_006468
86. Kbio3_001313
87. Cromoglicic Acid [who-dd]
88. Ninds_000857
89. Hy-b1619
90. Zinc1530788
91. Bdbm50440033
92. Mfcd00864784
93. Akos015961001
94. Db01003
95. 4h-1-benzopyran-2-carboxylic Acid, 5,5'-((2-hydroxytrimethylene)dioxy)bis(4-oxo-
96. Idi1_000857
97. Ethylenediaminedi-l-(+)-tartrate
98. Ncgc00021142-01
99. Ncgc00159523-01
100. Ac-12785
101. Bs-51003
102. Nci60_000229
103. Sbi-0051323.p003
104. Cs-0013552
105. Ft-0601561
106. 1,3-bis(2-carboxychromon-5-yloxy)propan-2-ol
107. C06928
108. D07753
109. E78436
110. 1,3-bis-(2-carboxychromon-5-yloxy)propan-2-ol
111. A922357
112. Q416427
113. J-009783
114. Brd-k20920669-304-06-7
115. 4h-1-benzopyran-2-carboxylic Acid, 5, {5'-[(2-hydroxytrimethylene)dioxy]bis(4-oxo-,}
116. 5,5'-((2-hydroxytrimethylene)dioxy)bis(4-oxo-4h-1-benzopyran-2-carboxylate)
117. 5,5'-(2-hydroxypropane-1,3-diyl)bis(oxy)bis(4-oxo-4h-chromene-2-carboxylic Acid)
118. 105878-73-7
119. 4h-1-benzopyran-2-carboxylic Acid, {5,5'-[(2-hydroxy-1,3-propanediyl)bis(oxy)]bis[4-oxo-,}
120. 4h-1-benzopyran-2-carboxylic Acid, 5,5'-((2-hydroxy-1,3-propanediyl)bis(oxy))bis(4-oxo-)
121. 5-[3-(2-carboxy-4-oxo-chromen-5-yl)oxy-2-hydroxy-propoxy]-4-oxo-chromene-2-carboxylic Acid
| Molecular Weight | 468.4 g/mol |
|---|---|
| Molecular Formula | C23H16O11 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 468.06926132 g/mol |
| Monoisotopic Mass | 468.06926132 g/mol |
| Topological Polar Surface Area | 166 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 835 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Asthmatic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A LARGE PROPORTION OF CHILDREN WITH CHRONIC, INTRACTABLE ASTHMA (MILD OR SEVERE) EXPERIENCE EITHER PARTIAL OR COMPLETE PROTECTION AFTER ORAL INHALATION OF CROMOLYN. /CROMOLYN DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 526
...EVIDENCE APPEARS TO INDICATE THAT PATIENTS WITH EXTRINSIC ASTHMA /KNOWN HYPERSENSITIVITY TO AN EXTRINSIC ALLERGEN/ ARE MORE LIKELY TO RESPOND TO CROMOLYN THAN PATIENTS WITH INTRINSIC ASTHMA /NO SUCH HYPERSENSITIVITY KNOWN/. HOWEVER, IT IS NOT CURRENTLY POSSIBLE TO PREDICT WHICH PATIENTS WILL RESPOND SATISFACTORILY TO CROMOLYN. /CROMOLYN DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 527
THE INHALATION OF CROMOLYN SHORTLY BEFORE EXERCISE LESSENS BRONCHOCONSTRICTION THAT SOME ASTHMATIC PATIENTS THEN DEVELOP. THIS EFFECT MAY BE ENHANCED IF PATIENT IS RECEIVING CONTINUOUS THERAPY. /CROMOLYN DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 526-7
For more Therapeutic Uses (Complete) data for CROMOLYN (20 total), please visit the HSDB record page.
ANAPHYLAXIS, VASCULITIS, OR OTHER SERIOUS ADVERSE EFFECTS HAVE NOT BEEN REPORTED IN MAN. URTICARIA & MACULOPAPULAR RASHES HAVE OCCURRED RARELY BUT HAVE CLEARED WHEN DRUG WAS WITHDRAWN. EOSINOPHILIC PNEUMONIA HAS BEEN ASSOCIATED WITH /INHALATION/ ADMIN OF CROMOLYN IN TWO PATIENTS. /DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 527
DESCRIBED IS A 42 YR OLD MALE PATIENTS WHO DEVELOPED SEVERE BRONCHOCONSTRICTION AFTER INHALING SODIUM CROMOGLYCATE (CROMOLYN).
PATERSON IC ET AL; BR MED J 2: (OCT) (1976)
A STUDY OF THE FREQUENCY OF ADVERSE REACTIONS (DERMATITIS, MYOSITIS, GASTROENTERITIS) TO CROMOLYN SODIUM, 0.5%, IN 375 ASTHMATIC PATIENTS, IS PRESENTED. THE FREQUENCY RATES WERE FOUND TO BE 2%; REACTIONS WERE NONLIFE THREATENING & COMPLETELY REVERSIBLE.
SETTIPANE GA ET AL; J AM MED ASSOC 241: (FEB) (1979)
SEVERE NASAL CONGESTION DEVELOPED IN 13 YR OLD ASTHMATIC PATIENTS AFTER 4 WK OF THERAPY WITH CROMOLYN & DISAPPEARED WITHIN 24 HR OF DISCONTINUANCE OF THERAPY. REACTION OCCURRED AGAIN WHEN REINITIATION OF CROMOLYN WAS ATTEMPTED 3 DAYS & 3 WEEKS AFTER FIRST ATTACK.
RAO M ET AL; J PEDIATR 86: (MAY) (1975)
For more Drug Warnings (Complete) data for CROMOLYN (25 total), please visit the HSDB record page.
For the management of patients with bronchial asthma. Also used in the treatment of vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
Cromoglicate or cromolyn (USAN), a synthetic compound, inhibits antigen-induced bronchospasms and, hence, is used to treat asthma and allergic rhinitis. Cromoglicate is used as an ophthalmic solution to treat conjunctivitis and is taken orally to treat systemic mastocytosis and ulcerative colitis.
Anti-Asthmatic Agents
Drugs that are used to treat asthma. (See all compounds classified as Anti-Asthmatic Agents.)
Mast Cell Stabilizers
Compounds that prevent the release of inflammatory mediators from MAST CELLS. (See all compounds classified as Mast Cell Stabilizers.)
S01GX01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07E - Intestinal antiinflammatory agents
A07EB - Antiallergic agents, excl. corticosteroids
A07EB01 - Cromoglicic acid
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AH - Agents for dermatitis, excluding corticosteroids
D11AH03 - Cromoglicic acid
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AC - Antiallergic agents, excl. corticosteroids
R01AC01 - Cromoglicic acid
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03B - Other drugs for obstructive airway diseases, inhalants
R03BC - Antiallergic agents, excl. corticosteroids
R03BC01 - Cromoglicic acid
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GX - Other antiallergics
S01GX01 - Cromoglicic acid
Absorption
1%
MOST OF ABSORBED DRUG IS EXCRETED UNCHANGED BY LIVER & KIDNEYS WITHIN A FEW DAYS & NONE APPEARS TO UNDERGO METABOLIC DEGRADATION. THE UNABSORBED PORTION (APPROX 80%) IS RECOVERABLE FROM FECES. FOLLOWING INHALATION, MAX PLASMA LEVEL...REACHED WITHIN SEVERAL MIN, & PLASMA T1/2 IS ONE TO ONE-HALF HOURS. /CROMOLYN DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 527
THE AMT OF CROMOLYN...ABSORBED INTO BLOODSTREAM FOLLOWING INHALATION OF A DOSE OF 20 MG DOES NOT APPEAR TO EXERT ANY GENERALIZED PHARMACOLOGIC EFFECTS. /CROMOLYN DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 527
THE SMALL AMT OF CROMOLYN THAT IS ABSORBED IS EXCRETED UNCHANGED BY LIVER & KIDNEYS... APPROX 10% OF A 20% MG DOSE...MAY REMAIN IN INHALER AFTER PATIENTS /USE/ &...APPROX 8% OF DOSE IS ABSORBED INTO BLOODSTREAM (PRIMARILY BY LUNG BUT ALSO BY GI TRACT). /CROMOLYN DISODIUM/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 527
FATE OF CROMOLYN WAS EXAMINED IN 12 ASTHMATIC PATIENTS. MAX PLASMA CONCN (MEAN 9.2 NG/ML OBTAINED WITHIN 15 MIN OF INHALING 20 MG). ABSORPTION FROM LUNG IS RAPID, MOST OF INHALED DOSE IS SWALLOWED.
WALKER SR ET AL; J PHARM PHARMACOL 24: (JUL) (1972)
For more Absorption, Distribution and Excretion (Complete) data for CROMOLYN (10 total), please visit the HSDB record page.
NO METABOLITES WERE DETECTED IN MAN & IN NINE MAMMALIAN SPECIES AFTER ORAL & IV ADMIN. /CROMOLYN DISODIUM/
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 268
1.3 hours
FATE OF CROMOLYN WAS EXAMINED IN 12 ASTHMATIC PATIENTS. AVG PLASMA T/2 WAS 81 MIN.
WALKER SR ET AL; J PHARM PHARMACOL 24: (JUL) (1972)
Cromoglicate inhibits degranulation of mast cells, subsequently preventing the release of histamine and slow-reacting substance of anaphylaxis (SRS-A), mediators of type I allergic reactions. Cromoglicate also may reduce the release of inflammatory leukotrienes. Cromoglicate may act by inhibiting calcium influx.
One important action of cromolyn is believed to be the inhibition of pulmonary mast cell degranulation in response to a variety of stimuli, including the interaction between cell-bound IgE and specific antigen. ... The release of histamine and other granular contents, as well as the production of leukotrienes, can be shown to be markedly reduced in vitro by cromolyn. However, its efficacy and potency are highly dependent on the source of the mast cells.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 668
... Attention has been focused on the ability of cromolyn to reverse various functional changes in leukocytes obtained from the blood of asthmatic subjects undergoing allergen challenge, such as increased expression of membrane-bound receptors.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 668
... Low concentrations (100 nM) of cromolyn can suppress completely the activation effects of chemoattractant peptides of human neutrophils, eosinophils, or monocytes.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 668
The mechanisms of action of cromolyn remain relatively poorly defined. Most attention has been focused on the ability of cromolyn to reduce the accumulation of intracellular Ca +2 induced by antigen in sensitized mast cells. One biochemical correlate of the reduction of histamine release from mast cells by cromolyn is the enhanced phosphorylation of a 78,000-dalton protein. Unfortunately, these observations have been made using rather high concn of cromolyn (50 to 200 uM), and their relationship to therapeutic response has yet to be established.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 668
For more Mechanism of Action (Complete) data for CROMOLYN (6 total), please visit the HSDB record page.
Global Sales Information

ABOUT THIS PAGE
50
PharmaCompass offers a list of Acido Cromoglicico API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acido Cromoglicico manufacturer or Acido Cromoglicico supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acido Cromoglicico manufacturer or Acido Cromoglicico supplier.
PharmaCompass also assists you with knowing the Acido Cromoglicico API Price utilized in the formulation of products. Acido Cromoglicico API Price is not always fixed or binding as the Acido Cromoglicico Price is obtained through a variety of data sources. The Acido Cromoglicico Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acido Cromoglicico manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acido Cromoglicico, including repackagers and relabelers. The FDA regulates Acido Cromoglicico manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acido Cromoglicico API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Acido Cromoglicico supplier is an individual or a company that provides Acido Cromoglicico active pharmaceutical ingredient (API) or Acido Cromoglicico finished formulations upon request. The Acido Cromoglicico suppliers may include Acido Cromoglicico API manufacturers, exporters, distributors and traders.
Acido Cromoglicico Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acido Cromoglicico GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acido Cromoglicico GMP manufacturer or Acido Cromoglicico GMP API supplier for your needs.
A Acido Cromoglicico CoA (Certificate of Analysis) is a formal document that attests to Acido Cromoglicico's compliance with Acido Cromoglicico specifications and serves as a tool for batch-level quality control.
Acido Cromoglicico CoA mostly includes findings from lab analyses of a specific batch. For each Acido Cromoglicico CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acido Cromoglicico may be tested according to a variety of international standards, such as European Pharmacopoeia (Acido Cromoglicico EP), Acido Cromoglicico JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acido Cromoglicico USP).
