

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDA Orange Book

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
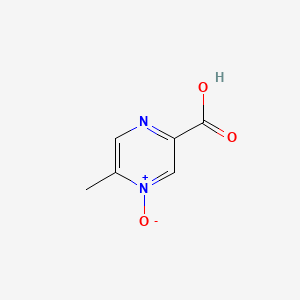

1. 5-methylpyrazine-2-carboxylic Acid 4-oxide
2. 5-methylpyrazinecarboxylic Acid 4-oxide
3. Nedios
4. Olbemox
5. Olbetam
1. 51037-30-0
2. Olbetam
3. 5-carboxy-2-methylpyrazine 1-oxide
4. Olbemox
5. 5-methylpyrazine-2-carboxylic Acid 4-oxide
6. 5-methylpyrazinecarboxylic Acid 4-oxide
7. 5-methyl-4-oxidopyrazin-4-ium-2-carboxylic Acid
8. 2-carboxy-5-methylpyrazine 4-oxide
9. K-9321
10. Nsc-759818
11. Chembl345714
12. Ncgc00160519-01
13. Dsstox_cid_26202
14. Dsstox_rid_81433
15. 5-methyl-4-oxido-2-pyrazin-4-iumcarboxylic Acid
16. Dsstox_gsid_46202
17. Cas-51037-30-0
18. Sr-05000001505
19. 5-methyl-2-pyrazinecarboxylic Acid 4-oxide
20. Acipimox-[d4]
21. Mfcd00865757
22. Acipimox (inn/ban)
23. Acipimox [inn]
24. Acipimox [mi]
25. Pyrazinecarboxylic Acid, 5-methyl-, 4-oxide
26. Acipimox [mart.]
27. K9ay9ir2sd
28. Acipimox [who-dd]
29. Schembl48922
30. Gtpl1596
31. Dtxsid2046202
32. Chebi:94688
33. Hms2090g05
34. Hms3655a14
35. Hms3713l18
36. Pharmakon1600-01504831
37. 5-carboxy-2-methylpyrazine1-oxide
38. Bcp28412
39. Hy-b0283
40. Zinc1481960
41. Tox21_111868
42. Tox21_112498
43. Tox21_112805
44. Ac-009
45. Bbl009924
46. Bdbm50208130
47. Nsc759818
48. S1806
49. Stk711089
50. 2-carboxy-5-methyl-pyrazine-4-oxide
51. Akos005530658
52. Ccg-213957
53. Db09055
54. Mb01535
55. 4-oxo-5-methyl-2-pyrazinecarboxylicacid
56. Ncgc00160519-02
57. Ncgc00160519-03
58. As-15565
59. Ba166874
60. 5-methyl-4-oxy-pyrazine-2-carboxylic Acid
61. Ft-0601589
62. M2053
63. Sw199084-2
64. D07190
65. Ab01274727-01
66. Ab01274727-02
67. Ab01274727_03
68. Ab01274727_04
69. K-9321;olbemox;olbetam; K9321; K 9321
70. A828409
71. 5-methyl-4-oxide-2-pyrazinecarboxylic Acid
72. 5-methylpyrazine-2-carboxylic Acid-4-oxide
73. Q2342544
74. Sr-05000001505-1
75. Sr-05000001505-2
76. W-105921
77. Brd-k63736853-001-01-4
| Molecular Weight | 154.12 g/mol |
|---|---|
| Molecular Formula | C6H6N2O3 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 154.03784206 g/mol |
| Monoisotopic Mass | 154.03784206 g/mol |
| Topological Polar Surface Area | 75.6 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 162 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of hyperlipidemias (abnormally elevated levels of any or all lipids and/or lipoproteins in the blood).
Hypolipidemic Agents
Substances that lower the levels of certain LIPIDS in the BLOOD. They are used to treat HYPERLIPIDEMIAS. (See all compounds classified as Hypolipidemic Agents.)
C10AD06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AD - Nicotinic acid and derivatives
C10AD06 - Acipimox
Acipimox inhibits the production of triglycerides by the liver and the secretion of VLDL, which leads indirectly to a modest reduction in LDL and increase in HDL.
Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
