

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
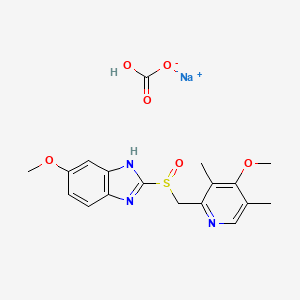

1. Acitrel
2. Omeprazole, Sodium Bicarbonate Drug Combination
3. Rapinex
4. San 05
5. Zegerid
1. 774595-73-2
2. Acitrel
3. Rapinex
4. Sodium;hydrogen Carbonate;6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1h-benzimidazole
5. Omeprazole/sodium Bicarbonate
6. Omeprazole / Sodium Bicarbonate
7. Omeprazole Mixture With Sodium Bicarbonate
8. Omeprazole, Sodium Bicarbonate Drug Combination
9. Schembl2523926
10. Dtxsid10998661
11. Sodium Hydrogen Carbonate--6-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methanesulfinyl]-1h-benzimidazole (1/1/1)
| Molecular Weight | 429.4 g/mol |
|---|---|
| Molecular Formula | C18H20N3NaO6S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 429.09705082 g/mol |
| Monoisotopic Mass | 429.09705082 g/mol |
| Topological Polar Surface Area | 157 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 487 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Proton Pump Inhibitors
Compounds that inhibit H(+)-K(+)-EXCHANGING ATPASE. They are used as ANTI-ULCER AGENTS and sometimes in place of HISTAMINE H2 ANTAGONISTS for GASTROESOPHAGEAL REFLUX. (See all compounds classified as Proton Pump Inhibitors.)
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
