


1. Bw 825c
2. Bw-825c
3. Bw825c
4. Semprex
1. 87848-99-5
2. Semprex
3. Acrivastin
4. Acrivastinum
5. Acrivastina
6. Bw 825c
7. Bw-825c
8. Bw A825c
9. Chebi:83168
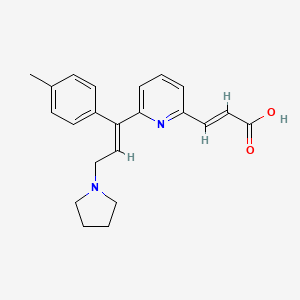
10. (e)-6-((e)-3-(1-pyrrolidinyl)-1-p-tolylpropenyl)-2-pyridineacrylic Acid
11. 2-propenoic Acid, 3-(6-(1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl)-2-pyridinyl)-, (e,e)-
12. A20f9xai7w
13. 2-propenoic Acid,3-[6-[(1e)-1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]-2-pyridinyl]-,(2e)-
14. (e)-3-[6-[(e)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridin-2-yl]prop-2-enoic Acid
15. Ncgc00182053-02
16. Acrivastinum [latin]
17. Acrivastina [spanish]
18. Acrivastine [usan:inn:ban]
19. (2e)-3-{6-[(1e)-1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl]pyridin-2-yl}acrylic Acid
20. Acrivastine (usan/inn)
21. Unii-a20f9xai7w
22. (e)-6-((e)-3-(1-pyrrolidinyl-1-p-tolylpropenyl)-2-pyridinacrylsaeure
23. Acrivastine [mi]
24. Acrivastine [inn]
25. Dsstox_cid_2555
26. Acrivastine [usan]
27. Acrivastine [vandf]
28. Schembl4702
29. Acrivastine [mart.]
30. Chembl1224
31. Dsstox_rid_76625
32. Dsstox_gsid_22555
33. Acrivastine [who-dd]
34. Mls006010115
35. Bidd:gt0209
36. Bw270c
37. Dtxsid6022555
38. Acrivastine, >=98% (hplc)
39. Acrivastine [orange Book]
40. Hms3886e20
41. Bcp06189
42. Hy-b1510
43. Zinc3776633
44. Tox21_113015
45. Ac-912
46. Bdbm50487466
47. Benadryl Allgy Relief Plus Decongest
48. Mfcd00869830
49. S5718
50. Akos005067182
51. Semprex-d Component Acrivastine
52. Cs-6454
53. Db09488
54. Ncgc00182053-03
55. (2e)-3-{6-[(1e)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-en-1-yl]pyridin-2-yl}prop-2-enoic Acid
56. Acrivastine Component Of Semprex-d
57. As-14623
58. Smr004701250
59. Cas-87848-99-5
60. D02760
61. D70156
62. 848a995
63. A916142
64. Q342745
65. Sr-01000942220
66. Q-200590
67. Sr-01000942220-1
68. (e)-3-{6-[3-pyrrolidino-1-(4-tolyl)prop-1e-enyl]-2-pyridyl}acrylic Acid
69. 6-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)-2-pyridineacrylic Acid
70. (e)-3-(6-((e)-3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridin-2-yl)acrylic Acid
71. 3-(6-(1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl)-2-pyridinyl)-
72. 2-propenoic Acid, 3-[6-[(1e)-1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propen-1-yl]-2-pyridinyl]-, (2e)-
| Molecular Weight | 348.4 g/mol |
|---|---|
| Molecular Formula | C22H24N2O2 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 348.183778013 g/mol |
| Monoisotopic Mass | 348.183778013 g/mol |
| Topological Polar Surface Area | 53.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 514 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the relief of symptoms associated with seasonal allergic rhinitis such as sneezing, rhinorrhea, pruritus, lacrimation, and nasal congestion.
FDA Label
Histamine H1 Antagonists, Non-Sedating
A class of non-sedating drugs that bind to but do not activate histamine receptors (DRUG INVERSE AGONISM), thereby blocking the actions of histamine or histamine agonists. These antihistamines represent a heterogenous group of compounds with differing chemical structures, adverse effects, distribution, and metabolism. Compared to the early (first generation) antihistamines, these non-sedating antihistamines have greater receptor specificity, lower penetration of BLOOD-BRAIN BARRIER, and are less likely to cause drowsiness or psychomotor impairment. (See all compounds classified as Histamine H1 Antagonists, Non-Sedating.)
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AX - Other antihistamines for systemic use
R06AX18 - Acrivastine
Absorption
Acrivastine was absorbed rapidly from the combination capsule following oral administration and was as bioavailable as a solution of acrivastine. After administration of SEMPREX-D Capsules, maximum plasma acrivastine concentrations were achieved at 1.14 0.23 hour.
Route of Elimination
A mass balance study in 7 healthy volunteers showed that acrivastine is primarily eliminated by the kidneys. Over a 72-hour collection period, about 84% of the administered total radioactivity was recovered in urine and about 13% in feces, for a combined recovery of about 97%.
Volume of Distribution
0.46 0.05 L/kg
Clearance
2.9 0.7 mL/min/kg
The mean terminal half-life for acrivastine was 1.9 0.3 hours following single oral doses and increased to 3.5 1.9 hours at steady state. The terminal half-life for the propionic acid metabolite was 3.8 1.4 hours.

LOOKING FOR A SUPPLIER?