
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 79-06-1
2. 2-propenamide
3. Prop-2-enamide
4. Propenamide
5. Acrylic Amide
6. Ethylenecarboxamide
7. Acrylic Acid Amide
8. Vinyl Amide
9. Akrylamid
10. Polyacrylamide
11. 2-propeneamide
12. Propeneamide
13. Acrylagel
14. Optimum
15. Poly(acrylamide)
16. Amresco Acryl-40
17. Propenoic Acid Amide
18. 9003-05-8
19. Akrylamid [czech]
20. Ethylene Carboxamide
21. Rcra Waste Number U007
22. Amid Kyseliny Akrylove
23. Acrylamide Polymer
24. Acrylamide Monomer
25. Acrylamide Solution
26. Amide Propenoic Acid
27. Polystolon
28. Polystoron
29. Porisutoron
30. Amid Kyseliny Akrylove [czech]
31. Acryl Amide
32. Flokonit E
33. Aminogen Pa
34. Acrylamide Monome
35. Flygtol Gb
36. Stipix Ad
37. Nsc 7785
38. Superfloc 84
39. Cytame 5
40. Polyhall 27
41. Sursolan P 5
42. Polyacrylamide Resin
43. Solvitose 433
44. Sumitex A 1
45. Superfloc 900
46. Cyanamer P 35
47. Gelamide 250
48. Nacolyte 673
49. Polyhall 402
50. Versicol W 11
51. Magnafloc R 292
52. Sumirez A 17
53. Sumirez A 27
54. Aerofloc 3453
55. Cyanamer P 250
56. Praestol 2800
57. Himoloc Ss 200
58. Sanpoly A 520
59. Chebi:28619
60. Stokopol D 2624
61. Bio-gel P 2
62. Reten 420
63. American Cyanamid Kpam
64. Biogel P-100
65. K-pam
66. Acrylamide Solution (50% Or Less)
67. American Cyanamid P-250
68. Dow Et 597
69. Taloflote
70. Pamid
71. 20r035klci
72. Acrylamide, Electrophoresis Grade
73. Nsc7785
74. Acrylamide Polymers
75. Acrylamide [un2074] [poison]
76. Acrylamide, Polymer
77. Nsc-7785
78. Pam (polymer)
79. Acrylamide, Polymers
80. Mfcd00008032
81. Acrylamide Homopolymer
82. Aam
83. Himoloc Ok 507
84. Percol 720
85. Paa 1 (homopolymer)
86. J 100 (polymer)
87. P 250 (polymer)
88. Dsstox_cid_27
89. K 4 (acrylic Polymer)
90. Paark 123sh
91. 2-propenamide, Homopolymer
92. Dsstox_rid_75328
93. Paa-1
94. Dow J 100
95. Dsstox_gsid_20027
96. Paa 70l
97. Pam-50
98. Q 41f
99. Tryptone
100. Ap 273
101. Et 597
102. Ccris 7
103. Acrylamide 1000 Microg/ml In Methanol
104. Cas-79-06-1
105. Hsdb 191
106. J 100
107. P 250
108. P 300
109. Propenoic Acid, Amide
110. Einecs 201-173-7
111. Un2074
112. Rcra Waste No. U007
113. Acrylarnide
114. Brn 0605349
115. Unii-20r035klci
116. Poly(acrylamide) Macromolecule
117. Ai3-04119
118. Amide Propenoate
119. Acryloic Acid Amide
120. 1hc
121. Acrylamide, 97%
122. Bio Gel P2
123. Polyacrylamide Solution
124. Acylamide-
125. Bio Gel P-2
126. Bio-gel P-2
127. Acrylamide (ultrapure)
128. Acrylamide [mi]
129. Ch2chconh2
130. Acrylamide [hsdb]
131. Acrylamide [iarc]
132. Acrylamide [inci]
133. Acrylamide, 53% Aqueous
134. Bmse000392
135. Acrylamide Solution, 40%
136. Acrylamide, >=98.0%
137. Acrylamide, >=99.9%
138. Ec 201-173-7
139. Acrylamide [mart.]
140. Wln: Zv1u1
141. Acrylamide-1,2,3-13c3
142. Acrylamide_ramanathangurudeeban
143. Bidd:er0629
144. Acrylamide, Analytical Standard
145. Chembl348107
146. Gtpl4553
147. Dtxsid5020027
148. Acrylamide, For Synthesis, 99%
149. Zinc901075
150. Bcp25183
151. Tox21_201526
152. Tox21_300145
153. Bdbm50226193
154. Nsc116573
155. Nsc116574
156. Nsc116575
157. Nsc118185
158. Stl282727
159. Acrylamide 100 Microg/ml In Methanol
160. Akos000120965
161. Acrylamide, Purum, >=98.0% (gc)
162. Nsc-116573
163. Nsc-116574
164. Nsc-116575
165. Nsc-118185
166. Un 2074
167. Acrylamide Monomer (ca. 50% In Water)
168. Acrylamide Monomer [for Electrophoresis]
169. Ncgc00090736-01
170. Ncgc00090736-02
171. Ncgc00090736-03
172. Ncgc00090736-04
173. Ncgc00090736-05
174. Ncgc00253932-01
175. Ncgc00259076-01
176. Acrylamide Monomer, [for Electrophoresis]
177. Acrylamide, Saj First Grade, >=98.0%
178. Db-124507
179. A0139
180. Acrylamide, Ultrapure, Electrophoresis Grade
181. Ft-0661414
182. Ft-0688081
183. Acrylamide, 30% Solution, Bisacrylamide Free
184. Acrylamide, 40% Solution, Bisacrylamide Free
185. C01659
186. Acrylamide, Suitable For Electrophoresis, >=99%
187. A839565
188. Acrylamide, For Electrophoresis, >=99.0% (gc)
189. Q342939
190. Acrylamide, For Molecular Biology, >=99% (hplc)
191. J-200356
192. J-510287
193. Acrylamide Solution, 40% In H2o, For Molecular Biology
194. Acrylamide, Certified Reference Material, Tracecert(r)
195. Acrylamide, For Electrophoresis, >=99% (hplc), Powder
196. Bc269f2e-d242-48e1-87e4-e51db86ff0a8
197. F8880-6341
198. Acrylamide Solution, 40%, For Electrophoresis, Sterile-filtered
199. Acrylamide, For Northern And Southern Blotting, Powder Blend
200. Acrylamide, Vetec(tm) Reagent Grade, Suitable For Electrophoresis
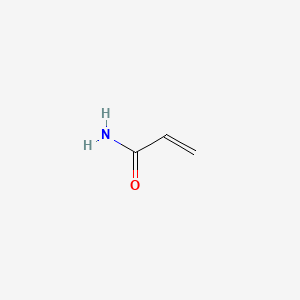
| Molecular Weight | 71.08 g/mol |
|---|---|
| Molecular Formula | C3H5NO |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 71.037113783 g/mol |
| Monoisotopic Mass | 71.037113783 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 57.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Acrylamide is well absorbed after oral, dermal, inhalational, and parenteral exposure, including through intact skin and mucous membranes. Efficient absorption of this compound is demonstrated by the observation that peak blood concentrations occur at approximately 1 hour after exposure. It is estimated that human elimination rates of acrylamide are only one-fifth that seen in rats.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1363
Acrylamide is primarily (90 to 95%) excreted in the urine as conjugated metabolite with less then 2% parent compound appearing in the urine. Smaller amounts of metabolites are also present in feces, bile, and other biological matrices, still with only small amounts being eliminated as unchanged parent. Acrylamide elimination is biphasic with an alpha half-life of less than 5 hours and a beta half-life of 6 to 8 days.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1364
In rats given 0.5-100 mg/kg bw of either (1-14(C))- or (2,3-14(C))acrylamide intravenously or orally, radioactivity was distributed rapidly throughout the body, with no selective accumulation in any tissue. Radioactivity was also distributed evenly among tissues of beagle dogs and miniature pigs
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V60 403 (1994)
... Can be absorbed through ... mucous membranes and lungs as well as the GI tract.
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 50
For more Absorption, Distribution and Excretion (Complete) data for ACRYLAMIDE (14 total), please visit the HSDB record page.
MICROSPHERES OF (14)C-LABELED POLYACRYLAMIDE WERE MAINLY (APPROX 80%) FOUND IN LIVER & SPLEEN BOTH AFTER IV & IP INJECTION IN MOUSE & RAT, ALSO DETECTED EARLY (1 HR AFTER IV INJECTION) IN BONE MARROW, & PARTICLE AGGREGATES WERE ALSO INITIALLY FOUND IN LUNGS.
SJOEHOLM I, EDMAN P; J PHARMACOL EXP THER 211(3) 656 (1979)
Acrylamide is primarily (90 to 95%) excreted in the urine as conjugated metabolite with less then 2% parent compound appearing in the urine. Smaller amounts of metabolites are also present in feces, bile, and other biological matrices, still with only small amounts being eliminated as unchanged parent. Acrylamide elimination is biphasic with an alpha half-life of less than 5 hours and a beta half-life of 6 to 8 days.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1364
Urinary metabolites among acrylamide-exposed animals were identified as N-acetyl-S- (3-amino-3-oxopropyl) cysteine (the N-acetyl-cysteine conjugate of acrylamide, following glutathione conjugation accounting for 67% of the total urinary metabolites found in rats, 41% of the total found in mice), N-acetyl-S- (3-amino-2-hydroxy-3-oxopropyl) cysteine (16% in rats, 21% in mice), N-acetyl-S- (1-carbamoyl-2-hydroxyethyl) cysteine (9% in rats, 12% in mice), glycidamide (6% in rats, 17% in mice), 2,3-dihydroxy-propionamide (2% in rats, 5% in mice), and a small amount of the parent compound (which was not possible to quantify).
European Chemicals Agency (ECHA); Registered Substances, Acrylamide (CAS Number: 79-06-1) (EC Number: 201-173-7) (Last updated: May 25, 2016). Available from, as of August 25, 2016: https://echa.europa.eu/
... In the present study, a low-dose of acrylamide (ACR; 18 mg/kg) was administered to male Wistar rats for 40 days. Ultra performance liquid chromatography/time of flight mass spectrometry (UPLC-Q-TOF MS) was used to examine urine samples from ACR-dosed and control animals. Multiple statistical analyses with principal component analysis (PCA) were used to investigate metabolite profile changes in urine samples, and to screen for potential neurotoxicity biomarkers. PCA showed differences between the ACR-dosed and control groups 20 days after the start of dosing; a bigger separation between the two groups was seen after dosing for 40 days. Levels of 4-guanidinobutanoic acid and 2-oxoarginine were significantly higher in urine from the ACR-dosed group than in urine from the control group after 10 days (p<0.05). Receiver operator characteristic (ROC) curve analysis suggested that 4-guanidinobutanoic acid and 2-oxoarginine were the major metabolites. Our results suggest that high levels of 4-guanidinobutanoic acid and 2-oxoarginine may be related to ACR neurotoxicity. These metabolites could, therefore, act as sensitive biomarkers for ACR exposure and be useful for investigating toxic mechanisms. They may also provide a scientific foundation for assessing the effects of chronic low-dose ACR exposure on human health.
PMID:25687561 Wang SY et al; Mol Biosyst 11 (4): 1146-55 (2015)
To study the toxic effect of chronic exposure to acrylamide (AA) at low-dose levels, we applied a metabolomics approach based on ultra-performance liquid chromatography/mass spectrometry (UPLC-MS). A total of 40 male Wistar rats were randomly assigned to different groups: control, low-dose AA (0.2 mg/kg bw), middle-dose AA (1 mg/kg bw) and high-dose AA (5 mg/kg bw). The rats continuously received AA via drinking water for 16 weeks. Rat urine samples were collected at different time points for measurement of metabolomic profiles. Thirteen metabolites, including the biomarkers of AA exposure (AAMA, GAMA and iso-GAMA), were identified from the metabolomic profiles of rat urine. Compared with the control group, the treated groups showed significantly increased intensities of GAMA, AAMA, iso-GAMA, vinylacetylglycine, 1-salicylate glucuronide, PE (20:1(11Z)/14:0), cysteic acid, L-cysteine, p-cresol sulfate and 7-ketodeoxycholic acid, as well as decreased intensities of 3-acetamidobutanal, 2-indolecarboxylic acid and kynurenic acid in rat urine. Notably, three new candidate biomarkers (p-cresol sulfate, 7-ketodeoxycholic acid and 1-salicylate glucuronide) in rat urine exposed to AA have been found in this study. The results indicate exposure to AA disrupts the metabolism of lipids and amino acids, induces oxidative stress.
PMID:27347750 Shi H et al; Xenobiotica 1-11 (2016) (Epub ahead of print)
For more Metabolism/Metabolites (Complete) data for ACRYLAMIDE (8 total), please visit the HSDB record page.
Plasma (animal studies): 2 days; whole body (animal studies): 6-18 days; [TDR, p. 40]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 40
Acrylamide elimination is biphasic with an alpha half-life of less than 5 hours and a beta half-life of 6 to 8 days.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1364
The distribution and metabolism of 2,3-(14)C-labeled acrylamide were studied in male rats. Three dose levels of acrylamide (1.0, 10, or 100 mg/kg) were administered orally. ... Elimination of the radiolabel from most tissues was biphasic with a terminal half-life of approx 8 days. The amount of (14)C in blood remained constant at 12% of the dose for about 7 days. However, (14)C in plasma was eliminated readily. The concn-time curve of parent acrylamide in tissues and blood fit a monoexponential curve with a half-life of approx 2 hr.
Miller MJ et al; Toxicol Appl Pharmacol 63 (1): 36-44 (1982)
Elimination from the tissues occurs in two phases: in the first phase its half-life is 5 hr, and in the second (delayed) phase, 8 days or less.
Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 947
The excretion half-life of parent acrylamide in rat urine was 7.8 hr. Using [1-14C]-acrylamide, ... /it was reported/ that approx 6% of the dose was exhaled as /carbon dioxide/. In an extensive study of the kinetics of both orally and iv admin [2,3-14C]-acrylamide, it was shown that the rate of elimination of the radiolabel in urine was independent of the route of admin. Within 24 hr, about 2/3 of the dose was excreted in the urine and 3/4 in 7 days. Fecal excretion was small (4.8% in 24 hr and 6% by 7 days). Since 15% of the dose appeared in the bile within 6 hr, acrylamide or its derivatives must undergo enterohepatic circulation. Thus, approx 80% of the radiolabel was excreted within 7 days and, of this, a very large proportion (90%) was in the form of metabolites.
WHO; Environ Health Criteria 49: Acrylamide p.41 (1985)
MICROSPHERES OF (14)C-LABELED POLYACRYLAMIDE WERE USED TO FOLLOW DISTRIBUTION & FATE IN MOUSE & RAT AFTER IV & IP INJECTION. PARTICLES WERE RAPIDLY CLEARED FROM CIRCULATION (T/2 IN RAT APPROX 40 MIN) BY MACROPHAGES OF RETICULOENDOTHELIAL SYSTEM.
SJOEHOLM I, EDMAN P; J PHARMACOL EXP THER 211(3) 656 (1979)
... In this study, we first investigated the effects of acrylamide (ACR) on slow axonal transport of neurofilaments in cultured rat dorsal root ganglia (DRG) neurons through live-cell imaging approach. Then for the underlying mechanisms exploration, the protein level of neurofilament subunits, motor proteins kinesin and dynein, and dynamitin subunit of dynactin in DRG neurons were assessed by western blotting and the concentrations of ATP was detected using the ATP Assay Kit. The results showed that ACR treatment results in a dose-dependent decrease of slow axonal transport of neurofilaments. Furthermore, ACR intoxication significantly increases the protein levels of the three neurofilament subunits (NF-L, NF-M, NF-H), kinesin, dynein, and dynamitin subunit of dynactin in DRG neurons. In addition, ATP level decreased significantly in ACR-treated DRG neurons. Our findings indicate that ACR exposure retards slow axonal transport of NF-M, and suggest that the increase of neurofilament cargoes, motor proteins, dynamitin of dynactin, and the inadequate ATP supply contribute to the ACR-induced retardation of slow axonal transport.
PMID:26721510 An L et al; Neurochem Res 41 (5): 1000-9 (2016)
Acrylamide produces a central-peripheral distal axonopathy when administered chronically. This is characterized functionally by decreases in the monosynaptic reflex and dorsal root potential and alterations in the characteristics of the dorsal root reflex. Acute administration of acrylamide inhibits the oxidative enzyme complex nicotinamide adenine dinucleotide (reduced form)-tetrazolium reductase and slows retrograde axoplasmic transport. This study was carried out to determine if the spinal cord reflexes are also affected following acute administration of acrylamide. Dose response studies revealed a dose-dependent increase in both the monosynaptic reflex and dorsal root reflex. A single injection of 50 mg/kg acrylamide caused an increase in both the monosynaptic reflex and dorsal root reflex within 15 min and continued for over 3 hr. These data are paradoxical since chronic administration of acrylamide results in decreased function. Two possible mechanisms are proposed. First, calcium ion regulation may be involved in both the acute and chronic effects of acrylamide on spinal cord reflexes. Second, a depolarization of the neurons is occurring just prior to cell injury or death.
PMID:3715927 Goldstein BD, Fincher DR; Toxicol Lett 31 (2): 93-100 (1986)
ANALYTICAL

ABOUT THIS PAGE
14
PharmaCompass offers a list of Acrylamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acrylamide manufacturer or Acrylamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acrylamide manufacturer or Acrylamide supplier.
PharmaCompass also assists you with knowing the Acrylamide API Price utilized in the formulation of products. Acrylamide API Price is not always fixed or binding as the Acrylamide Price is obtained through a variety of data sources. The Acrylamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acrylamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acrylamide, including repackagers and relabelers. The FDA regulates Acrylamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acrylamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Acrylamide supplier is an individual or a company that provides Acrylamide active pharmaceutical ingredient (API) or Acrylamide finished formulations upon request. The Acrylamide suppliers may include Acrylamide API manufacturers, exporters, distributors and traders.
Acrylamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acrylamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acrylamide GMP manufacturer or Acrylamide GMP API supplier for your needs.
A Acrylamide CoA (Certificate of Analysis) is a formal document that attests to Acrylamide's compliance with Acrylamide specifications and serves as a tool for batch-level quality control.
Acrylamide CoA mostly includes findings from lab analyses of a specific batch. For each Acrylamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acrylamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Acrylamide EP), Acrylamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acrylamide USP).
