
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
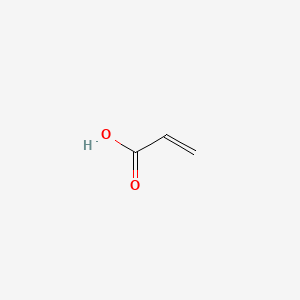

1. 2-propenoic Acid
2. Acrylate
3. Acrylic Acid, Aluminum Salt
4. Acrylic Acid, Ammonium Salt
5. Acrylic Acid, Ca (2:1) Salt
6. Acrylic Acid, Ca (2:1) Salt, Dihydrate
7. Acrylic Acid, Cobalt (2+) Salt
8. Acrylic Acid, Fe (3+) Salt
9. Acrylic Acid, Magnesium Salt
10. Acrylic Acid, Potassium Salt
11. Acrylic Acid, Silver Salt
12. Acrylic Acid, Sodium Salt
13. Acrylic Acid, Zinc Salt
14. Hystoacril
15. Magnesium Acrylate
16. Vinylformic Acid
1. 2-propenoic Acid
2. 79-10-7
3. Propenoic Acid
4. Prop-2-enoic Acid
5. Vinylformic Acid
6. Acroleic Acid
7. Ethylenecarboxylic Acid
8. Propene Acid
9. Polyacrylic Acid
10. Propenoate
11. Acrylate
12. Polyacrylate
13. Glacial Acrylic Acid
14. Acrylic Acid, Glacial
15. Kyselina Akrylova
16. 9003-01-4
17. Rcra Waste Number U008
18. Carbopol 934p
19. Nsc 4765
20. Carbopol 940
21. Acrylic Acid, Polymer
22. J94pbk7x8s
23. Acrylic Polymer
24. 2-propenoic Acid, Homopolymer
25. Acrylic Resin
26. Aron
27. Antiprex A
28. Chebi:18308
29. Versicol E9
30. Nsc4765
31. Acrylic Acid Resin
32. Acrysol Ase-75
33. C3:1n-1
34. Versicol E 7
35. Versicol E15
36. Nsc-4765
37. Acrysol A 1
38. Acrysol A 3
39. Acrysol A 5
40. Acrysol A-1
41. Acrysol Ac 5
42. Carbopol 960
43. Carboset 515
44. Primal Ase 60
45. Revacryl A191
46. Versicol K 11
47. Versicol S 25
48. Viscalex Hv 30
49. Dispex C40
50. Acrysol Ws-24
51. Cyguard 266
52. Joncryl 678
53. Jurimer Ac 10h
54. Jurimer Ac 10p
55. Nalfloc 636
56. Carboxy Vinyl Polymer
57. Good-rite K 37
58. Revacryl A 191
59. 25987-55-7
60. Junlon 110
61. Polyacrylate Elastomers
62. Propenoic Acid Polymer
63. Viscon 103
64. Good-rite K 702
65. Good-rite K 732
66. Acrylic Acid, Polymers
67. Good-rite Ws 801
68. Ncgc00166246-01
69. Acrylic Acid Homopolymer
70. Polymerized Acrylic Acid
71. Synthemul 90-588
72. Aron A 10h
73. Atactic Poly(acrylic Acid)
74. Carboset Resin No. 515
75. Old 01
76. Pa 11m
77. Paa-25
78. Caswell No. 009a
79. Acrylates
80. P 11h
81. P-11h
82. Ws 24
83. Acide Acrylique
84. Acido Acrilio
85. Acido Acrilio [spanish]
86. Acide Acrylique [french]
87. Ws 801
88. Kyselina Akrylova [czech]
89. Calcium Polyacrylate
90. R968
91. Ccris 737
92. Hsdb 1421
93. Einecs 201-177-9
94. Un2218
95. Rcra Waste No. U008
96. Unii-j94pbk7x8s
97. Allenediol
98. Brn 0635743
99. Ai3-15717
100. Acrysol Lmw-20x
101. Xpa
102. Acrylic Acid Homopolymer Calcium Salt
103. Acrlylic Acid
104. Dow Latex 354
105. Acrylic Acid, Inhibited
106. Ch2=chcooh
107. Dsstox_cid_28
108. (stabilized With Mehq)
109. Carbomer 934 (nf)
110. Carbomer 940 (nf)
111. Carbomer 941 (nf)
112. Carbopol 910 (tn)
113. Carbopol 934 (tn)
114. Carbopol 940 (tn)
115. Carbopol 941 (tn)
116. 2-propenoic Acid, Homopolymer, Calcium Salt
117. Carbomer 934p (nf)
118. Carbopol 934p (tn)
119. Carbomer 910 (usan)
120. Acrylic Acid [mi]
121. Carbomer 1342 (nf)
122. Carbopol 1342 (tn)
123. Ec 201-177-9
124. Acrylic Acid [hsdb]
125. Acrylic Acid [iarc]
126. Acrylic Acid [inci]
127. Dsstox_rid_79425
128. Wln: Qv1u1
129. Dsstox_gsid_39229
130. Araldite Gt 7004 Acrylate
131. Polyacrylic Acid Calcium Salt
132. 4-02-00-01455 (beilstein Handbook Reference)
133. Un 2218 (salt/mix)
134. Acrylic Acid, P.a., 99%
135. Chembl1213529
136. Dtxsid0039229
137. Zinc895281
138. Str00040
139. Tox21_112372
140. Lmfa01030193
141. Mfcd00004367
142. Nsc106034
143. Nsc106035
144. Nsc106036
145. Nsc106037
146. Nsc112122
147. Nsc112123
148. Nsc114472
149. Nsc165257
150. Nsc226569
151. Stl281870
152. Akos000118799
153. Db02579
154. Nsc-106034
155. Nsc-106035
156. Nsc-106036
157. Nsc-106037
158. Nsc-112122
159. Nsc-112123
160. Nsc-114472
161. Nsc-165257
162. Nsc-226569
163. Cas-79-10-7
164. Bp-30259
165. A0141
166. Ft-0621875
167. Ft-0660730
168. C00511
169. C19501
170. D03392
171. D03393
172. D03394
173. D03395
174. D03396
175. D03397
176. Acrylic Acid Contains 200ppm Mehq As Inhibitor
177. Acrylic Acid, Inhibited [un2218] [corrosive]
178. A830860
179. Q324628
180. Z57127944
181. F0001-2070
182. 2-propenoic Acid, Block Polymer With Sulfonated Ethenylbenzene
183. Acrylic Acid, Anhydrous, Contains 200 Ppm Mehq As Inhibitor, 99%
184. Acrylic Acid, Saj First Grade, >=97.0%, Contains 190-210 Ppm Mehq As Stabilizer
185. Block Copolymer Of Sulfonated Polystyrene And Polyacrylic Acid (mw 250,000)
186. 55927-87-2
| Molecular Weight | 72.06 g/mol |
|---|---|
| Molecular Formula | C3H4O2 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 72.021129366 g/mol |
| Monoisotopic Mass | 72.021129366 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 55.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tissue Adhesives
Substances used to cause adherence of tissue to tissue or tissue to non-tissue surfaces, as for prostheses. (See all compounds classified as Tissue Adhesives.)
Groups of three male Sprague-Dawley rats were given a single oral dose of 4, 40, or 400 mg/kg of 2,3-(14)C-acrylic acid or 2, 20, or 200 mg/kg 2,3-(14)C-ethyl acrylate in 0.5% aqueous methylcellulose (25 uCi/kg) at a volume of 10 mL/kg ... Urine, feces, and expired carbon dioxide were collected at various intervals up to 72 hours after dosing, and the animals were then killed. Acrylic acid and ethyl acrylate were eliminated rapidly, primarily in expired carbon dioxide (44% to 65%). 35% to 60% of the acrylic acid and approximately 60% of the ethyl acrylate was eliminated within 8 hours. Urinary excretion of radioactive metabolites was greater with ethyl acrylate. Within 72 hours, 90% to 76% of the radioactivity was recovered from the animals dosed with 4 and 400 mg/kg acrylic acid; 19% to 25% was recovered in the tissues, with most being found in adipose tissue, (9% to 15%). With ethyl acrylate, 108% to 73% of the dose was recovered with 2 to 200 mg/kg; 13% to 10% was found in the tissues, with the most generally being found in muscle tissue (5.6% to 5%), and 28% to 8% was excreted in the urine.
PMID:12537929 Zondlo Fiume M; Int J Toxicol 21 (Suppl 3): 1-50 (2002)
Three fasted male Sprague-Dawley rats were given 400 mg/kg 1,2,3-(13)C3-acrylic acid coadministered with 2,3-(14)C-acrylic acid (40 to 46 uCi/kg) in distilled water by gavage ... Urine, feces, and expired air were collected for 72 hours, and the animals were then killed. Total recovery was 98%. The majority of the radioactivity, 78%, was recovered in expired carbon dioxide. Approximately 13% of the radioactivity was recovered in the tissues, with almost 5% of the dose found in the muscle, 3% found in the liver, 2% found in the skin, and 1% found in adipose tissue. The tissue-to-blood radioactivity concentration ratios were 11.1, 3.2, 2.6, 2.4, 2.1, and 2.0 for the liver, kidneys, adipose tissue, stomach, spleen, and large intestine, respectively. Approximately 6% of the dose was eliminated in the urine and 1% was eliminated in the feces. Nuclear magnetic resonance spectroscopy did not detect unchanged acrylic acid in the urine.
PMID:12537929 Zondlo Fiume M; Int J Toxicol 21 (Suppl 3): 1-50 (2002)
The disposition of (14)C-acrylic acid was determined in vitro using clipped dorsal skin from male rats ... One percent (v/v) (14)C-Acrylic Acid, 95 uL, was applied to the exposed epidermal surface (1.77 sq cm), and an evaporation trap was fitted over the skin. Over a 6-hour period, 23.9% +/- 5.4% of the dose was absorbed in the effluent or was found in the skin and at least 60% of the dose was evaporated. Total recovery of the applied dose was approximately 85%.
PMID:12537929 Zondlo Fiume M; Int J Toxicol 21 (Suppl 3): 1-50 (2002)
Acrylic acid is rapidly absorbed in rats and mice after oral or inhalation administration. A hybrid computational fluid dynamics and physiologically-based pharmacokinetics inhalation dosimetry model was constructed for interspecies (rat-human) extrapolation of acrylic acid tissue dose in the olfactory region of the nasal cavity. The model simulations indicate that under similar exposure conditions human olfactory epithelium is exposed with acrylic acid to 2-3 fold lower than rat olfactory epithelium. After dermal administration some acrylic acid is evaporated, the remainder undergoes rapid absorption in these animals. Dermal absorption is strongly dependent on the vehicle and the pH value of the solution.
European Chemicals Bureau; Risk Assessment for Acrylic acid (CAS No 79-10-7) Final Report p.56 (2002)
For more Absorption, Distribution and Excretion (Complete) data for Acrylic acid (11 total), please visit the HSDB record page.
Acrylic acid is rapidly metabolized by oxidative pathways to CO2. The main metabolic pathway of acrylic acid seems to be a secondary, non-vitamin-B12 dependent pathway of propionic acid metabolism consisting in reactions similar to fatty acid beta-oxidation. In urine poorly characterized substances of a higher polarity than those of acrylic acid are detected. Unmetabolized acrylic acid could not be detected in urine, however small amounts of 3-hydroxypropionic acid were found. Epoxide intermediates were not detected. In vitro (stomach tissue) and in vivo acrylic acid reacts with glutathione and non-protein sulfhydryls to a very low extent. High dosages of acrylic acid leading to tissue damage cause the formation of small amounts of mercapturic acid derivates.
European Chemicals Bureau; Risk Assessment for Acrylic acid (CAS No 79-10-7) Final Report p.56 (2002)
After oral administration of 4, 40, or 400 mg/kg bw [2,3-(14)C]-acrylic acid in a 0.5% aqueous methylcellulose solution to rats, within 72 hr 44-65% of the radioactivity had been eliminated via expired air and 2.9-4.3% remained in the urine. The HPLC profile of metabolites observed in the urine of rats indicated two major metabolites. One of the major metabolites co-eluted was 3-hydroxypropionic acid. Radioactivity could not be detected at the retention times corresponding to that of 2,3-epoxypropionic acid or N-acetyl-S-(2-carboxy-2-hydroxyethyl)cysteine. One hour following an oral dose of acrylic acid (4, 40, 400, or 1,000 mg/kg) in rats a significant depletion of /Non-protein sulfhydryls/ (NPSH) in the glandular stomach was reported at doses above 4 mg/kg. In the forestomach NPSH depletion occurred at a dose of 1,000 mg/kg. No significant effect of acrylic acid on NPSH in the blood or liver was observed
European Chemicals Bureau; Risk Assessment for Acrylic acid (CAS No 79-10-7) Final Report p.54 (2002)
... The metabolites of acrylic acid and propionic acid /were compared/ using (13)C-NMR analysis of the urine of rats after gavage of single doses (400 mg/kg bw). 3-Hydroxypropionic acid, N-acetyl-S-(2-carboxyethyl)cysteine and N-acetyl-S-(2-carboxyethyl)cysteine-S-oxide were identified as metabolites of acrylic acid. No unchanged acrylic acid was detected. In contrast, the spectra of urine from a propionic acid-treated rat revealed only a few minor (13)C-enriched signals that were assigned to methylmalonic acid. These metabolites (CO2 and methylmalonic acid) are consistent with the known major vitamin B12-dependent pathway of propionate metabolism in mammals. An alternative pathway involves beta-oxidation. Acrylyl-CoA forms 3-hydroxypropionic acid that can then be oxidized to malonic semialdehyde. Further catabolism yields acetyl-CoA and CO2. It is conceivable that excretion and detection of the mercapturates are a consequence of the high dose used in this experiment.
European Chemicals Bureau; Risk Assessment for Acrylic acid (CAS No 79-10-7) Final Report p.54 (2002)
Following single doses (40 or 150 mg/kg) of [1-(14)C]-acrylic acid to rats urinary metabolites and tissues were analyzed by HPLC. A major polar metabolite which could not be identified accounted for approximately 2 to 3% of the dose. A metabolite that coeluted with 3-hydroxypropionic acid was also detected. Small amounts of several other metabolites were detected. Plasma and liver from orally dosed rats were also analyzed for acrylic acid and metabolites by HPLC. One hour after dosing, a metabolite in plasma that co-eluted with 3-hydroxypropionic acid accounted for about 0.5% of the dose after 40 mg/kg bw. This metabolite was also detected in plasma after application of the higher dose. Neither acrylic acid nor metabolites were detected in plasma or liver at times later than 1 hr. They were not detected in kidney at any time after administration ... In other experiments, livers from mice dosed by gavage following a similar dosing regime were analyzed for acrylic acid and metabolites by HPLC. Several metabolites of higher polarity than those of acrylic acid including 3-hydroxypropionic acid were detected 1 hr after administration, but not at times later than 1 hr. Acrylic acid was not detected in livers from mice at any time after cutaneous administration of 40 mg/kg bw. After cutaneous dosing in rats, a peak that coeluted with acrylic acid was detected in urine along with the major metabolite found after oral dosing. A trace amount of another metabolite was detected in urine from the 40 mg/kg bw cutaneous dose group but not after dosing 10 mg/kg bw.
European Chemicals Bureau; Risk Assessment for Acrylic acid (CAS No 79-10-7) Final Report pp.54-5 (2002).
For more Metabolism/Metabolites (Complete) data for Acrylic acid (13 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

ABOUT THIS PAGE
56
PharmaCompass offers a list of Acrylic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Acrylic Acid manufacturer or Acrylic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Acrylic Acid manufacturer or Acrylic Acid supplier.
PharmaCompass also assists you with knowing the Acrylic Acid API Price utilized in the formulation of products. Acrylic Acid API Price is not always fixed or binding as the Acrylic Acid Price is obtained through a variety of data sources. The Acrylic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acrylic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acrylic Acid, including repackagers and relabelers. The FDA regulates Acrylic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acrylic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Acrylic Acid supplier is an individual or a company that provides Acrylic Acid active pharmaceutical ingredient (API) or Acrylic Acid finished formulations upon request. The Acrylic Acid suppliers may include Acrylic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Acrylic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acrylic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Acrylic Acid active pharmaceutical ingredient (API) in detail. Different forms of Acrylic Acid DMFs exist exist since differing nations have different regulations, such as Acrylic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acrylic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Acrylic Acid USDMF includes data on Acrylic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acrylic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acrylic Acid suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Acrylic Acid Drug Master File in Japan (Acrylic Acid JDMF) empowers Acrylic Acid API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Acrylic Acid JDMF during the approval evaluation for pharmaceutical products. At the time of Acrylic Acid JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Acrylic Acid suppliers with JDMF on PharmaCompass.
Acrylic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acrylic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acrylic Acid GMP manufacturer or Acrylic Acid GMP API supplier for your needs.
A Acrylic Acid CoA (Certificate of Analysis) is a formal document that attests to Acrylic Acid's compliance with Acrylic Acid specifications and serves as a tool for batch-level quality control.
Acrylic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Acrylic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acrylic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Acrylic Acid EP), Acrylic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acrylic Acid USP).
