
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
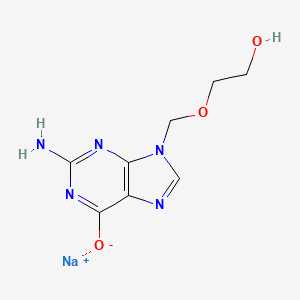

1. 9-((2-hydroxyethoxy)methyl)guanine
2. Aci Sanorania
3. Aci-sanorania
4. Acic
5. Aciclobeta
6. Aciclostad
7. Aciclovir
8. Aciclovir Alonga
9. Aciclovir Sanorania
10. Aciclovir Von Ct
11. Aciclovir-sanorania
12. Acifur
13. Acipen Solutab
14. Acivir
15. Activir
16. Acyclo V
17. Acyclo-v
18. Acycloguanosine
19. Acyclovir
20. Alonga, Aciclovir
21. Antiherpes Creme
22. Avirax
23. Cicloferon
24. Clonorax
25. Cusiviral
26. Genvir
27. Herpetad
28. Herpofug
29. Herpotern
30. Herpoviric
31. Isavir
32. Laciken
33. Mapox
34. Maynar
35. Milavir
36. Opthavir
37. Sodium, Acyclovir
38. Solutab, Acipen
39. Supraviran
40. Viclovir
41. Vipral
42. Virax Puren
43. Virax-puren
44. Viraxpuren
45. Virherpes
46. Virmen
47. Virolex
48. Virupos
49. Virzin
50. Wellcome 248u
51. Wellcome-248u
52. Wellcome248u
53. Zoliparin
54. Zovirax
55. Zyclir
1. Aciclovir Sodium
2. 69657-51-8
3. Sodium Acyclovir
4. Sodium 2-((2-amino-6-oxo-1h-purin-9(6h)-yl)methoxy)ethanolate
5. Acyclovir Sodium Salt
6. Bw 248u Sodium
7. Mfcd01694138
8. Bw-248u Sodium
9. Bw-248u-sodium
10. Sodium 2-amino-9-((2-hydroxyethoxy)methyl)-9h-purin-6-olate
11. Sodium 2-amino-9-[(2-hydroxyethoxy)methyl]purin-6-olate
12. 9-((2-hydroxyethoxy)methyl)guanine Monosodium Salt
13. Aciclovir Natrium
14. Bw 248u
15. Zovirax Sterile Powder
16. 927l42j563
17. Acycloguanosine Sodium (obs.)
18. Unii-927l42j563
19. 6h-purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-, Monosodium Salt
20. Schembl40722
21. Chembl1200380
22. Bcp13172
23. Akos015896105
24. 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6h-purin-6-one Monosodium Salt
25. As-12705
26. Sy027836
27. O12074
28. Q27271485
29. Sodium2-amino-9-((2-hydroxyethoxy)methyl)-9h-purin-6-olate
30. 6h-purin-6-one, 1,9-dihydro-2-amino-9-((2-hydroxyethoxy)methyl)-, Monosodium Salt
| Molecular Weight | 247.19 g/mol |
|---|---|
| Molecular Formula | C8H10N5NaO3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 247.06813348 g/mol |
| Monoisotopic Mass | 247.06813348 g/mol |
| Topological Polar Surface Area | 122 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 237 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Acyclovir sodium |
| Drug Label | Acyclovir is a synthetic nucleoside analog active against herpes viruses. Acyclovir for Injection USP is a sterile lyophilized powder for intravenous administration only. Each 500 mg vial contains 500 mg of acyclovir and 49 mg of sodium, and each 100... |
| Active Ingredient | Acyclovir sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Aurobindo Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Acyclovir sodium |
| Drug Label | Acyclovir is a synthetic nucleoside analog active against herpes viruses. Acyclovir for Injection USP is a sterile lyophilized powder for intravenous administration only. Each 500 mg vial contains 500 mg of acyclovir and 49 mg of sodium, and each 100... |
| Active Ingredient | Acyclovir sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Aurobindo Pharma |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.

GDUFA
DMF Review : Reviewed
Rev. Date : 2015-02-20
Pay. Date : 2013-09-26
DMF Number : 10945
Submission : 1994-06-09
Status : Active
Type : II

NDC Package Code : 46014-1019
Start Marketing Date : 2016-12-16
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN PRESCRIPTION COMPOUNDING

| Available Reg Filing : ASMF |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24289
Submission : 2010-10-15
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29995
Submission : 2016-11-28
Status : Active
Type : II
Date of Issue : 2016-07-06
Valid Till : 2019-07-06
Written Confirmation Number : WC-0378
Address of the Firm :


GDUFA
DMF Review : Reviewed
Rev. Date : 2020-10-06
Pay. Date : 2020-08-26
DMF Number : 35112
Submission : 2020-08-31
Status : Active
Type : II

NDC Package Code : 14335-150
Start Marketing Date : 2021-12-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11829
Submission : 1996-01-24
Status : Inactive
Type : II





 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2015-02-20
Pay. Date : 2013-09-26
DMF Number : 10945
Submission : 1994-06-09
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11829
Submission : 1996-01-24
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2020-10-06
Pay. Date : 2020-08-26
DMF Number : 35112
Submission : 2020-08-31
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24289
Submission : 2010-10-15
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29995
Submission : 2016-11-28
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2016-07-06
Valid Till : 2019-07-06
Written Confirmation Number : WC-0378
Address of the Firm : Plot No. 100 Sector 56 Phase IV, HSIIDC Kundli, District Sonepat Haryana India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 46014-1019
Start Marketing Date : 2016-12-16
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

NDC Package Code : 14335-150
Start Marketing Date : 2021-12-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
About the Company : Established in 1990, Inabata France, a part of the Inabata Group, used to export chemical and pharmaceutical products to Japan. In 2006, it acquired Pharmasynthèse. Today, Inabata...
About the Company : Arihantanam Life Care Pvt. Ltd. is engaged in Manufacturing of Various Active Pharmaceuticals Ingredients Since 2006. We are a WHO-GMP approved facility and have installed moder...

About the Company : Fareva’s API division has special technologies for high potent APIs (HPAPIs) down to the OEB-6 level, aseptic crystallization (sterile APIs), Spray Drying etc, with volumes rangi...

About the Company : Rajasthan Antibiotics Limited (RAL-Life) is a fast track integrated pharmaceutical company which commenced its commercial production in 1991 and has grown in size and stature to b...

About the Company : Shouyuan chemical (one of the leading chemicals supplier in China) specializes in manufacturing, supplying, and custom synsthesis latest chemicals. Our products cover all kinds of ...

About the Company : Established in 2002, Tecoland represents selected cGMP manufacturers with proven capabilities in organic synthesis, fermentation production as well as process and method developmen...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ACYCLOVIR
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 50MG BASE/ML
Packaging :
Approval Date : 1999-07-26
Application Number : 75114
Regulatory Info : DISCN
Registration Country : USA
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 50MG BASE/ML
Packaging :
Approval Date : 2020-06-17
Application Number : 207919
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : ACYCLOVIR SODIUM INJECTION
Dosage Form : SOLUTION
Dosage Strength : 50MG/ML
Packaging :
Approval Date :
Application Number : 2494558
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1GM BASE/VIAL
Packaging :
Approval Date : 1997-04-22
Application Number : 74596
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Packaging :
Approval Date : 1997-04-22
Application Number : 74758
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : INJECTION
Dosage Strength : 250MG
Packaging :
Approval Date :
Application Number : 18603
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 200MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Acyclovir
Dosage Form : Aciclovir 8% 100Ml Oral Use
Dosage Strength : os suspe 100 ml 8%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : CLOVIR
Dosage Form : TABLET
Dosage Strength : 400MG
Packaging :
Approval Date :
Application Number : 202168
Regulatory Info : Generic
Registration Country : India

Regulatory Info : DISCN
Registration Country : USA
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1GM BASE/VIAL
Packaging :
Approval Date : 2017-03-29
Application Number : 203927
Regulatory Info : DISCN
Registration Country : USA

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 50MG BASE/ML
Approval Date : 1999-07-26
Application Number : 75114
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 50MG BASE/ML
Approval Date : 2020-06-17
Application Number : 207919
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1GM BASE/VIAL
Approval Date : 1998-02-27
Application Number : 74897
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1GM BASE/VIAL
Approval Date : 1997-04-22
Application Number : 74596
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Approval Date : 1997-04-22
Application Number : 74596
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ZOVIRAX
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 250MG BASE/VIAL **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1983-08-30
Application Number : 18603
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 500MG BASE/VIAL
Approval Date : 1997-10-15
Application Number : 74913
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1GM BASE/VIAL
Approval Date : 1997-10-15
Application Number : 74913
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1GM BASE/VIAL
Approval Date : 2016-02-29
Application Number : 205771
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : ACYCLOVIR SODIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 50MG BASE/ML
Approval Date : 2018-08-31
Application Number : 206535
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Euclivir
Dosage Form :
Dosage Strength : Cream Derm 10 G 5%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Aciclin
Dosage Form :
Dosage Strength : Cream Derm 10 G 5%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Zovirax
Dosage Form : Acyclovir 800Mg 35 Joined' Oral Use
Dosage Strength : 35 CPR 800 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Zovirax
Dosage Form : Acyclovir 500Mg 5 Units Parenteral Use
Dosage Strength : 5 bottles EV 500 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Acyclovir
Dosage Form : Acyclovir 400Mg 25 Units' Oral Use
Dosage Strength : 25 CPR 400 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Acyclovir
Dosage Form : Acyclovir 250Mg 5 Units Parenteral Use
Dosage Strength : 5 VIALS EV 250 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Acyclovir
Dosage Form : Acyclovir 800Mg 35 Joined' Oral Use
Dosage Strength : 35 CPR 800 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Cycloviran
Dosage Form : Acyclovir 200Mg 25 Units' Oral Use
Dosage Strength : 25 CPR 200 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Aciclovir Mylan
Dosage Form : POWDER FOR SOLUTION FOR INFUSION
Dosage Strength : 25 MG / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Regulatory Info :
Registration Country : Italy
Brand Name : Acyclovir
Dosage Form : Acyclovir 800Mg 35 Joined' Oral Use
Dosage Strength : 35 CPR 800 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : ACYCLOVIR SODIUM INJECTION
Dosage Form : SOLUTION
Dosage Strength : 50MG/ML
Packaging :
Approval Date :
Application Number : 2494558
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : ACYCLOVIR SODIUM INJECTION
Dosage Form : SOLUTION
Dosage Strength : 50MG/ML
Packaging : 10/20ML
Approval Date :
Application Number : 2236926
Regulatory Info : Prescription
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Dry Powder for Injecti...
Dosage Strength : 500MG
Packaging : Vial
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : Vial
Regulatory Info :
Dosage : Dry Powder for Injecti...
Dosage Strength : 500MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Cream
Dosage Strength : 5%
Packaging : 5gm
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 5gm
Regulatory Info : Generic
Dosage : Cream
Dosage Strength : 5%
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : INJECTION
Dosage Strength : 500MG
Packaging :
Approval Date :
Application Number : 74885
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 500MG
Brand Name :
Approval Date :
Application Number : 74885
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 400MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 400MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 500mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 500mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 200MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 200MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 800MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 800MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : CLOVIR
Dosage Form : TABLET
Dosage Strength : 800MG
Packaging :
Approval Date :
Application Number : 20089
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : TABLET
Dosage Strength : 800MG
Brand Name : CLOVIR
Approval Date :
Application Number : 20089
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : CLOVIR
Dosage Form : TABLET
Dosage Strength : 200MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : TABLET
Dosage Strength : 200MG
Brand Name : CLOVIR
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name : CLOVIR
Dosage Form : TABLET
Dosage Strength : 400MG
Packaging :
Approval Date :
Application Number : 202168
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : TABLET
Dosage Strength : 400MG
Brand Name : CLOVIR
Approval Date :
Application Number : 202168
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?