Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Amphetamine Sulfate
2. Amphetamine Sulfate (2:1)
3. Centramina
4. Desoxynorephedrin
5. Fenamine
6. L-amphetamine
7. Levo Amphetamine
8. Levo-amphetamine
9. Levoamphetamine
10. Phenamine
11. Phenopromin
12. Sulfate, Amphetamine
13. Thyramine
1. 1-phenylpropan-2-amine
2. Dl-amphetamine
3. 300-62-9
4. 1-phenyl-2-aminopropane
5. Norephedrane
6. Alpha-methylphenethylamine
7. Fenopromin
8. Phenedrine
9. Elastonon
10. Beta-aminopropylbenzene
11. 1-methyl-2-phenylethylamine
12. 1-phenyl-2-propylamine
13. Actedron
14. Allodene
15. Anorexide
16. Anorexine
17. Benzebar
18. Benzolone
19. Isoamyne
20. Mecodrin
21. Novydrine
22. Oktedrin
23. Ortedrine
24. Percomon
25. Profamina
26. Simpatina
27. Adipan
28. Isomyn
29. Finam
30. Protioamphetamine
31. Alpha-methylbenzeneethaneamine
32. 1-phenyl-2-propanamine
33. 3-phenyl-2-propylamine
34. Psychedrine
35. Amfetaminum
36. Fenylo-izopropylaminyl
37. (phenylisopropyl)amine
38. Dl-alpha-methylphenethylamine
39. 2-amino-1-phenylpropane
40. Racemic-desoxynor-ephedrine
41. Beta-phenylisopropylamin
42. Amfetamin
43. Dyanavel
44. Dl-benzedrine
45. Rac-amphetamine
46. .beta.-aminopropylbenzene
47. (+-)-alpha-methylphenylethylamine
48. 1-phenyl-2-amino-propan
49. Adzenys Er
50. Benzeneethanamine, .alpha.-methyl-
51. Amphetamine, Dl-
52. .alpha.-methylbenzeneethanamine
53. 60-15-1
54. Beta-phenylisopropylamine
55. Nsc-27159
56. (+/-)-desoxynorephedrine
57. Dl-1-phenyl-2-aminopropane
58. Ck833kgx7e
59. Dexedrine
60. Amfetamine (inn)
61. Dexacaps
62. Amfetamine [inn]
63. Phenethylamine, .alpha.-methyl-, (.+/-.)-
64. Benzeneethanamine, .alpha.-methyl-, (.+/-.)-
65. (+-)-benzedrine
66. Amfetamina [italian]
67. Anfetamina [spanish]
68. Dea No. 1100
69. Amphetamin
70. Amfetamine [inn:ban]
71. Amfetaminum [inn-latin]
72. Adderall Xr
73. Amphetamine Salts
74. Dyanavel Xr
75. Amfetamina [inn-spanish]
76. Beta-aminopropylbenzene (van)
77. D-am
78. Delcobese
79. Fenylo-izopropylaminyl [polish]
80. Benzeneethanamine, Alpha-methyl-
81. (+-)-alpha-methylphenethylamine
82. 1-phenyl-2-aminopropane (van)
83. Benzeneethanamine, Alpha-methyl-, (+-)-
84. D-1-phenyl-2-aminopropan
85. Hsdb 3287
86. Beta-phenylisopropylamin [german]
87. D-1-phenyl-2-aminopropane
88. D-2-amino-1-phenylpropane
89. (+-)-alpha-methylbenzeneethanamine
90. 1-phenyl-2-amino-propan [german]
91. Amphetamine Resin Complex
92. Beta-phenyl-isopropylamine
93. Einecs 200-458-3
94. Einecs 206-096-2
95. Unii-ck833kgx7e
96. Phenethylamine, Alpha-methyl-, (+-)-
97. (+)-.alpha.-methylphenethylamine
98. Adderal
99. Isoamycin
100. Phenethylamine, Alpha-methyl-
101. Amphetamine-
102. Ai3-02438
103. 3-amino-1-propylbenzene
104. Amfetamin (tn)
105. Dyanavel (tn)
106. Component Of Amodex
107. Phenethylamine, D-
108. S(+)-amphetamine
109. Adzenys (tn)
110. (plusmn)-amphetamine
111. Benzeneethanamine, .alpha.-methyl-, (s)-
112. Noclon (salt/mix)
113. Norephedrine, Deoxy-
114. Fenamin (salt/mix)
115. Ortenal (salt/mix)
116. Zedrine (salt/mix)
117. (+/-)-benzedrine
118. Euphodyn (salt/mix)
119. Stimulan (salt/mix)
120. Fabedrine (salt/mix)
121. Oraldrina (salt/mix)
122. Vapedrine (salt/mix)
123. Amphetamine Mixture With Dextroamphetamine
124. Sympametin (salt/mix)
125. Component Of Biphetamine
126. Phenethylamine, (+)-
127. 3-phenylpropan-2-amine
128. Amphetamine [mi]
129. (.+/-.)-benzedrine
130. Benzeneethanamine, (s)-
131. .beta.-phenylisopropylamin
132. Alpha-methyl Phenethylamine
133. Amphetamine, (d)
134. Amphetamine [hsdb]
135. Alpha-methylphenylethylamine
136. Chembl405
137. .beta.-phenylisopropylamine
138. Amphetamine [vandf]
139. Amfetamine [mart.]
140. Schembl8858
141. .alpha.-methylphenethylamine
142. Amfetamine [who-dd]
143. Oprea1_447423
144. D/l-amphetamine Hydrochloride
145. (.+/-.)-desoxynorephedrine
146. .alpha.-methylphenylethylamine
147. Divk1c_000991
148. Wln: Zy1&1r
149. (s)-.alpha.-phenylethylamine
150. D-.alpha.-methylphenethylamine
151. Chebi:2679
152. Gtpl4804
153. Wln: Zy1&1r -d
154. .alpha.-methylbenzeneethaneamine
155. Dl-.alpha.-methylphenethylamine
156. Dtxsid4022600
157. Hms503g03
158. Kbio1_000991
159. Phenethylamine, .alpha.-methyl-
160. (+/-)-beta-phenylisopropylamine
161. Amphetamine [orange Book]
162. Chebi:132233
163. Ninds_000991
164. Rac-(2r)-1-phenylpropan-2-amine
165. (+)-.alpha.-methylphenylethylamine
166. Nsc27159
167. Nsc73713
168. .alpha.-methylphenethylamine, D-form
169. Bdbm50005246
170. (s)-(+)-.beta.-phenylisopropylamine
171. Amphetamine, Its Salts, Optical Isomers, And Salts Of Its Optical Isomers
172. (.+/-.)-.beta.-phenylisopropylamine
173. Ab07478
174. Db00182
175. Nt-0201
176. (.+/-.)-.alpha.-methylphenethylamine
177. Idi1_000991
178. Rac-amphetamine 1.0 Mg/ml In Methanol
179. (.+/-.)-.alpha.-methylphenylethylamine
180. Db-047697
181. (+/-)-.alpha.-methylphenethylamine
182. C07514
183. D03740
184. D07445
185. Mydayis (mixed Salts Of A Single Entity)
186. L000864
187. Q179452
188. Benzeneethanamine, .alpha.-methyl-, (+/-)-
189. Selegiline Hydrochloride Impurity B [ep Impurity]
190. Dl-amphetamine Solution, 1 Mg/ml In Methanol, Drug Standard
191. (+/-)-amphetamine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
192. (+/-)-amphetamine Solution, 100 Mug/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
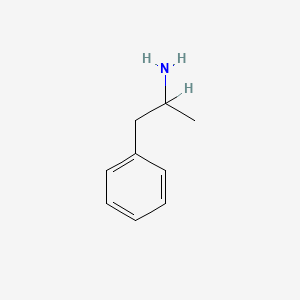
| Molecular Weight | 135.21 g/mol |
|---|---|
| Molecular Formula | C9H13N |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 135.104799419 g/mol |
| Monoisotopic Mass | 135.104799419 g/mol |
| Topological Polar Surface Area | 26 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 84.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic Agents; Adrenergic Uptake Inhibitors; Central Nervous System Stimulants; Dopamine Agents; Dopamine Uptake Inhibitors; Sympathomimetics
National Library of Medicine's Medical Subject Headings. Amphetamine. Online file (MeSH, 2015). Available from, as of November 23, 2015: https://www.nlm.nih.gov/mesh/2015/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Amphetamine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of September 30, 2015: https://clinicaltrials.gov/search/intervention=Amphetamine
Evekeo (amphetamine sulfate tablets, USP) is indicated for: 1. Narcolepsy 2. Attention Deficit Disorder with Hyperactivity as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children with behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate to severe distractibility, short attention span, hyperactivity, emotional lability , and impulsivity. The diagnosis of the syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or not be warranted. 3. Exogenous Obesity as a short term (a few weeks) adjunct in a regimen of weight reduction based on caloric restriction for patients refractory to alternative therapy, e.g., repeated diets, group programs, and other drugs. /Included in US product label/
NIH; DailyMed. Current Medication Information for EVEKEO- amphetamine sulfate tablet (Revised: November 2015). Available from, as of November 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb12811f-4cce-4010-aa48-d17c46ca1d3a
Vet: to alleviate anesthetic overdosage, particularly with barbiturates. To incr ... response to external stimuli such as depressive states & milk fever in cows. May have value in selected cases of encephalomyelitis (horses) & epileptic (cattle) or hyperkinetic syndromes.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 17
/BOXED WARNING/ Amphetamines have a high potential for abuse. Administration of amphetamines for prolonged periods of time may lead to drug dependence and must be avoided. Particular attention should be paid to the possibility of subjects obtaining amphetamines for non-theraputic use or distribution to others, and the drugs should be prescribed or dispensed sparingly. Misuse of amphetamine may cause sudden death and serious cardiovascular adverse events.
NIH; DailyMed. Current Medication Information for EVEKEO- amphetamine sulfate tablet (Revised: November 2015). Available from, as of November 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb12811f-4cce-4010-aa48-d17c46ca1d3a
Amphetamines are distributed into milk in concentrations 3-7 times maternal blood concentrations. A decision should be made whether to discontinue nursing or the drug.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2530
Amphetamines should be used during pregnancy only if the potential benefits justify the possible risks to the fetus. During pregnancy it is questionable whether potential benefits from amphetamines outweigh potential risks. Infants born to women dependent on amphetamines have an increased risk of prematurity, low birthweight, and withdrawal symptoms (e.g., dysphoria, lassitude, agitation).
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2529
Adverse effects of amphetamines may include nervousness, insomnia, irritability, talkativeness, changes in libido, dizziness, headaches, increased motor activity, chilliness, pallor or flushing, blurred vision, mydriasis, and hyperexcitability. Exacerbation of motor or phonic tics, Tourette's syndrome, dyskinesia, seizures, euphoria, dysphoria, emotional lability, and impotence have been reported in patients receiving amphetamines. Psychotic episodes have occurred rarely in patients receiving amphetamines at recommended dosages.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2528
For more Drug Warnings (Complete) data for AMPHETAMINE (21 total), please visit the HSDB record page.
In adults, 120 mg of amphetamine has caused death, but in one patient 200 mg produced only mild signs of peripheral sympathomimetic activity. Death usually is preceded by seizures and coma and usually results from cardiovascular collapse or from seizures.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2530
The acute lethal dose in adults has been reported at 20-25 mg/kg, and in children, 5 mg/kg. Death from as little as 1.5 mg/kg in an adult has also been noted.
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 348
Toxic amphetamine blood concentration: 50 ug/dL; Lethal amphetamine blood concentration: 200 ug/dL /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 420
Amphetamine is indicated for the treatment of attention-deficit/hyperactivity disorders (ADHD) as well as for the treatment of central nervous system disorders such as narcolepsy. ADHD is a complex disorder associated with the substantial heterogeneity in etiology, clinical presentation, and treatment outcome. ADHD comes from a complex interplay between interdependent genetic and non-genetic factors which cause complex mental disorders in children and teenagers. On the other hand, narcolepsy is a chronic sleep disorder typically resenting during adolescence and characterized by excessive daytime sleepiness. Amphetamine is also being used nowadays off-label for the treatment of obesity, depression and chronic pain.
FDA Label
From its mechanism of action, it has been demonstrated that amphetamine augments the concentration of noradrenaline in the prefrontal cortex and dopamine in the striatum on a dose and time-dependent manner. The indistinct release of neurotransmitters which include adrenaline is known to produce cardiovascular side effects. There are old reports of a cognitive enhancement related to the administration of amphetamine in which improvements in intelligence test scores were reported. In ADHD, amphetamine has been largely showed to produce remarkable improvements in school performance, behavior, and demeanor. The effect was shown to be produced through both racemic forms and to this date, the use of racemic forms 3:1 (D:L) is very common. The therapeutic effect of amphetamine on serotonin does not seem to have a significant clinical effect on ADHD as observed on comparative studies with amphetamine and fenfluramine, a powerful serotonin releasing factor. However, the indirect effect on serotonin might have an effect on the depression and anxiety profile of ADHD. Studies regarding the illicit use of amphetamine in which heavy consumers were studied proved the generation of a paranoid state which flagged this drug as a psychiatric danger compound. This observation was supported by the continuous reports of misuse in patients under depression.
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Dopamine Agents
Any drugs that are used for their effects on dopamine receptors, on the life cycle of dopamine, or on the survival of dopaminergic neurons. (See all compounds classified as Dopamine Agents.)
Dopamine Uptake Inhibitors
Drugs that block the transport of DOPAMINE into axon terminals or into storage vesicles within terminals. Most of the ADRENERGIC UPTAKE INHIBITORS also inhibit dopamine uptake. (See all compounds classified as Dopamine Uptake Inhibitors.)
Adrenergic Agents
Drugs that act on adrenergic receptors or affect the life cycle of adrenergic transmitters. Included here are adrenergic agonists and antagonists and agents that affect the synthesis, storage, uptake, metabolism, or release of adrenergic transmitters. (See all compounds classified as Adrenergic Agents.)
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BA - Centrally acting sympathomimetics
N06BA01 - Amfetamine
Absorption
Amphetamine is well absorbed in the gut and as it is a weak base hence the more basic the environment the more of the drug is found in a lipid-soluble form and the absorption through lipid-rich cell membranes is highly favored. The peak response of amphetamine occurs 1-3 hours after oral administration and approximately 15 minutes after injection and it presents a bioavailability of over 75%. Complete amphetamine absorption is usually done after 4-6 hours.
Route of Elimination
The elimination of amphetamine is mainly via the urine from which about 40% of the excreted dose is found as unchanged amphetamine. About 90% of the administered amphetamine is eliminated 3 days after oral administration. The rate of elimination of amphetamine highly depends on the urine pH in which acidic pH will produce a higher excretion of amphetamine and basic pH produces a lower excretion.
Volume of Distribution
Amphetamine is reported to have a high volume of distribution of 4 L/kg.
Clearance
The reported normal clearance rate is of 0.7 L.h/kg. This clearance has been shown to get significantly reduced in patients with renal impairment reaching a value of 0.4 L.h/kg.
Children: Children eliminated amphetamine faster than adults.
Drug Facts and Comparisons 2015. Clinical Drug Information, LLC St. Louis, MO 2015, p. 1302
/MILK/ Amphetamines are excreted in human milk.
NIH; DailyMed. Current Medication Information for EVEKEO- amphetamine sulfate tablet (Revised: November 2015). Available from, as of November 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb12811f-4cce-4010-aa48-d17c46ca1d3a
Amphetamines are readily absorbed from the GI tract and effects persist for 4-24 hours. Amphetamines are distributed into most body tissues with high concentrations occurring in the brain and CSF. Amphetamine appears in the urine within about 3 hours following oral administration. Urinary excretion of the amphetamines is pH-dependent and excretion is enhanced in acidic urine. Following oral administration of racemic amphetamine to humans, approximately equal amounts of both isomers were excreted during the first 12 hours; after the first 12 hours, a continually decreasing proportion of the d-isomer was excreted.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2531
Amphetamine has been measured in human sweat at a median range of 15.5 (low dose 6.5-40.5) and 53.8 (high dose 34.0-83.4) ng per patch(1).
(1) Daughton CG, Ruhoy IS; Environ Toxicol Chem 28(12): 2495-521 (2009)
For more Absorption, Distribution and Excretion (Complete) data for AMPHETAMINE (7 total), please visit the HSDB record page.
Amphetamine is known to be metabolized by the liver under the action of the CYP2D6. The metabolic pathway of amphetamine is mainly defined by aromatic hydroxylation, aliphatic hydroxylation, and n-dealkylation. The formed metabolites in this pathway are 4-hydroxyamphetamine, 4-hydroxynorephedrine, hippuric acid, benzoic acid, benzyl methyl ketone, and p-hydroxyamphetamine which is known to be a potent hallucinogen. However, a significant part of the original compound remains unchanged.
Amphetamine is metabolized in the liver by aromatic hyroxylation, N-dealkylation, and deamination. Although the enzymes involved in amphetamine metabolism have not been clearly defined, cytochrome P450 (CYP-450) 2D6 is known to be involved with formation of 4-hydroxy-amphetamine. Because CYP2D6 is genetically polymorphic, population variations in amphetamine metabolism are a posibility.
Drug Facts and Comparisons 2015. Clinical Drug Information, LLC St. Louis, MO 2015, p. 1302
Metabolism that results in aromatic hydroxylation, aliphatic hydroxylation, and n-dealkylation of amphetamines can give rise to active metabolites such as the potent hallucinogen p-hydroxyamphetamine. Other metabolic pathways, including deamination and subsequent side chain oxidation, produce inactive amphetamine derivatives.
Haddad, L.M. (Ed). Clinical Management of Poisoning and Drug Overdose 3rd Edition. Saunders, Philadelphia, PA. 1998., p. 563
Amphetamine is a known human metabolite of Fenproporex.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of amphetamine highly depends on the isomer. For d-amphetamine, the reported half-life is of approximately 9-11 hours while for l-amphetamine the half-life is reported to be of 11-14 hours. The urine pH can modify this pharmacokinetic parameter which can vary from 7 hours in acid urine to 34 hours for alkaline urine.
Biological half-life is between 10-13 hr in adults and 9-11 hr in children.
Drug Facts and Comparisons 2015. Clinical Drug Information, LLC St. Louis, MO 2015, p. 1302
It is important to consider that amphetamine has a very similar structure to the catecholamine neurotransmitters mainly on the presence of a long planar conformation, the presence of an aromatic ring and nitrogen in the aryl side chain. Amphetamine, as well as other catecholamines, is taken into presynaptic nerve terminals by the association with two sodium ions and one chloride ion. The complex of the amphetamine with the ions is actively transported by monoamine reuptake transporters. As amphetamine acts competitively with the endogenous monoamines, the greater the number of amphetamines the more internalized amphetamine will be found. Once inside the presynaptic terminal, amphetamine displaces other monoamines to be stored by VMAT2 which produces the pumping of the neurotransmitters into the synapse by a process called retro-transport. This process of release of neurotransmitters is approximately fourfold more potent in the d-isomer for the release of dopamine. The mechanism of action of amphetamine is complemented by the inhibition of the reuptake and of monoamine oxidase which acts synergistically to produce a significant increase the monoamine concentration. This activity is not done as an inhibitor per se but more as a competitive substrate and thus, amphetamine is known to be a weak dopamine reuptake inhibitor, moderate noradrenaline reuptake inhibitor and very weak serotonin reuptake inhibitor. From this specific action, the l-isomer is known to be significantly less potent. Lastly, amphetamine is known to be an inhibitor of the mitochondrial-bound enzyme MAO which is the catalytic enzyme in charge of degrading all the excess of neurotransmitters. This mechanism of action is often overpassed as amphetamine is a weak MAO inhibitor but this mechanism cannot be dismissed.
Inactivation of sympathomimetic noncatecholamines largely depends on breakdown by monoamine oxidase and since substitution of an alkyl group for hydrogen on the a-carbon atom blocks enzymatic inactivation of the amino group, the duration of action of noncatecholamines (but not of catecholamines, which are inactivated largely by a different mechanism) is prolonged by a-substitution. The absence of a hydroxyl group on the aromatic ring of amphetamine reduces inactivation of the drug in the GI tract and the amphetamines are active following oral administration.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2531
ABOUT THIS PAGE
36
PharmaCompass offers a list of Norephedrane API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Norephedrane manufacturer or Norephedrane supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Norephedrane manufacturer or Norephedrane supplier.
PharmaCompass also assists you with knowing the Norephedrane API Price utilized in the formulation of products. Norephedrane API Price is not always fixed or binding as the Norephedrane Price is obtained through a variety of data sources. The Norephedrane Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Norephedrane manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Norephedrane, including repackagers and relabelers. The FDA regulates Norephedrane manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Norephedrane API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Norephedrane supplier is an individual or a company that provides Norephedrane active pharmaceutical ingredient (API) or Norephedrane finished formulations upon request. The Norephedrane suppliers may include Norephedrane API manufacturers, exporters, distributors and traders.
Norephedrane Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Norephedrane GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Norephedrane GMP manufacturer or Norephedrane GMP API supplier for your needs.
A Norephedrane CoA (Certificate of Analysis) is a formal document that attests to Norephedrane's compliance with Norephedrane specifications and serves as a tool for batch-level quality control.
Norephedrane CoA mostly includes findings from lab analyses of a specific batch. For each Norephedrane CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Norephedrane may be tested according to a variety of international standards, such as European Pharmacopoeia (Norephedrane EP), Norephedrane JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Norephedrane USP).

