
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
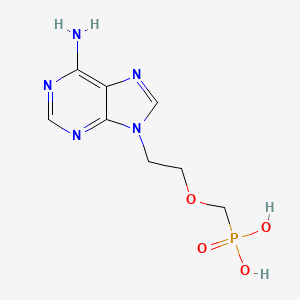

1. 9-(2-phosphonomethoxyethyl)adenine
2. 9-(2-phosphonylmethoxyethyl)adenine
3. 9-pmea
1. 106941-25-7
2. Pmea
3. ((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)phosphonic Acid
4. 9-(2-phosphonylmethoxyethyl)adenine
5. Gs 0393
6. Gs-0393
7. 9-(2-(phosphonomethoxy)ethyl)adenine
8. Gs 393
9. Drg-0156
10. N-(2-phosphonylmethoxyethyl)adenine
11. {[2-(6-amino-9h-purin-9-yl)ethoxy]methyl}phosphonic Acid
12. 2-(6-aminopurin-9-yl)ethoxymethylphosphonic Acid
13. Hsdb 8079
14. Chembl484
15. Phosphonic Acid, [[2-(6-amino-9h-purin-9-yl)ethoxy]methyl]-
16. 6gqp90i798
17. 106941-25-7 (free Acid)
18. 9-[2-(phosphonomethoxy)ethyl]adenine
19. (2-(6-amino-9h-purin-9-yl)ethoxy)methylphosphonic Acid
20. Ncgc00160539-01
21. Phosphonic Acid, ((2-(6-amino-9h-purin-9-yl)ethoxy)methyl)-
22. [2-(6-amino-9h-purin-9-yl)ethoxy]methylphosphonic Acid
23. Brn 3561094
24. [[2-(6-amino-9h-purin-9-yl)ethoxy]methyl]phosphonic Acid
25. Adefovir [usan:inn:ban]
26. Unii-6gqp90i798
27. N-(2-phophonomethoxyethyl-2,6-diaminopurine)
28. Adefovir(1-)
29. Mfcd00866943
30. Adefovir [usan]
31. Adefovir (usan/inn)
32. Adefovir [inn]
33. Adefovir [mi]
34. Adefovir [vandf]
35. Adefovir [mart.]
36. Amd3100 And Pmea
37. Adefovir [who-dd]
38. Dsstox_cid_26214
39. Dsstox_rid_81442
40. Dsstox_gsid_46214
41. Schembl49373
42. Chebi:2469
43. Adefovir, >=98% (hplc)
44. Dtxsid6046214
45. Chebi:134512
46. P-[[2-(6-amino-9h-purin-9-yl)ethoxy]methyl]phosphonic Acid
47. 9-[2-phosphonomethoxyethyl]adenine
48. Bcp22955
49. Hy-b1826
50. 9-(2-phosphorylmethoxyethyl)adenine
51. Tox21_111882
52. Ac-012
53. Bdbm50001103
54. S5068
55. Zinc21297308
56. 9-[(2-phosphonylmethoxy)ethyl]adenine
57. Akos005259246
58. Db13868
59. Ks-5018
60. Ncgc00160539-02
61. Ba166179
62. Cas-106941-25-7
63. Cs-0013877
64. Ft-0601785
65. 9-[3-(phosphonomethoxy)ethyl]adenine (pmea)
66. D02768
67. 941a257
68. A801540
69. Q353551
70. Sr-01000945034
71. Q-200594
72. Sr-01000945034-1
73. Pmea, Gs-393, 9-(2-phosphonylmethoxyethyl)adenine
74. [2-(6-amino-purin-9-yl)-ethoxymethyl]-phosphonic Acid
75. ((2-(6-amino-9h-purin-9-yl)ethoxy)-methyl)phosphonic Acid
76. (pmea)[2-(6-amino-purin-9-yl)-ethoxymethyl]-phosphonic Acid
77. [2-(6-amino-purin-9-yl)-ethoxymethyl]-phosphonic Acid(pmea)
78. Hydrogen {[2-(6-amino-9h-purin-9-yl)ethoxy]methyl}phosphonate
79. P-[[2-(6-amino-9h-purin-9-yl)ethoxy]methyl]-phosphonic Acid
80. 5hg
| Molecular Weight | 273.19 g/mol |
|---|---|
| Molecular Formula | C8H12N5O4P |
| XLogP3 | -2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 273.06269088 g/mol |
| Monoisotopic Mass | 273.06269088 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Phosphonic Acids; Adenine/analogs & derivatives; Antiviral Agents; Reverse Transcriptase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
Adefovir is indicated for the treatment of chronic hepatitis B in patients 12 years of age and older with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. This indication is based on histological, virological, biochemical, and serological responses in adult patients with HBeAg+ and HBeAg- chronic hepatitis B with compensated liver function, and with clinical evidence of lamivudine-resistant hepatitis B virus with either compensated or decompensated liver function. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
For patients 12 to less than 18 years of age, the indication is based on virological and biochemical responses in patients with HBeAg+ chronic hepatitis B virus infection with compensated liver function. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
This study investigated the efficacy, safety, and pharmacokinetics of adefovir dipivoxil (ADV) in children and adolescents with chronic hepatitis B (CHB). A total of 173 treatment-naive and treatment-experienced children with hepatitis B e antigen (HBeAg)+ CHB were randomized to ADV or placebo. Randomization was stratified by age (2 to <7 years; >7 to <12 years; >12 to <18 years) and prior treatment. Significantly more ADV-treated subjects aged 12 to <18 years achieved the primary efficacy endpoint (serum hepatitis B virus [HBV] DNA <1,000 copies/mL and normal alanine aminotransferase) compared to placebo-treated subjects (23% versus 0%; P = 0.007). In the younger groups, differences between ADV and placebo at the end of blinded treatment were not statistically significant. More ADV-treated subjects had HBeAg seroconversion: 18 of 113 (15.9%) versus three of 57 (5.3%) (but P = 0.051), and more met the combined endpoint of HBeAg seroconversion, HBV DNA <1,000 copies/mL and normal alanine aminotransferase (12/113 versus 0/57; P = 0.009). No subject developed an ADV-associated mutation that has been linked to HBV DNA rebound (that is, mutations rtN236T or rtA181V). ADV plasma concentrations were comparable across groups and within the target range. ADV treatment was well tolerated; no new safety issues were identified. Treatment-related adverse events were reported for 12% of ADV-treated and 10% of placebo-treated subjects. After 48 weeks of ADV treatment, antiviral efficacy in subjects ages 12 to <18 years with HBeAg+ CHB was similar to that observed in a study in adult treatment-naive subjects with HBeAg+ CHB. ADV was not different from placebo in subjects aged 2 to 11 years despite adequate plasma ADV exposure in all three age groups. CONCLUSION: ADV showed significant antiviral efficacy in subjects aged 12 to 17 years with HBeAg+ CHB, but was not different from placebo in subjects aged 2 to 11 years.
PMID:18433023 Jonas MM et al; Hepatology 47 (6): 1863-71 (2008)
/BOXED WARNING/ Severe acute exacerbations of hepatitis have been reported in patients who have discontinued anti-Hepatitis B therapy including adefovir. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-Hepatitis B therapy. If appropriate, resumption of anti-Hepatitis B therapy may be warranted.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
/BOXED WARNING/ In patients at risk of or having underlying renal dysfunction, chronic administration of adefovir may result in nephrotoxicity. These patients should be monitored closely for renal function and may require dose adjustment.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
/BOXED WARNING/ HIV resistance may emerge in chronic hepatitis B patients with unrecognized or untreated Human Immunodeficiency Virus (HIV) infection treated with anti-hepatitis B therapies, such as therapy with adefovir, that may have activity against HIV.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with other antiretrovirals.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
For more Drug Warnings (Complete) data for Adefovir (20 total), please visit the HSDB record page.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
Following oral administration of adefovir dipivoxil, approximate bioavailability of adefovir is 59%. A single 10-mg oral dose of adefovir dipivoxil in adults results in peak adefovir plasma concentration within 0.58-4 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 805
4% or less of adefovir is bound to plasma or serum proteins.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 805
In vitro binding of adefovir to human plasma or human serum proteins is less than or equal to 4% over the adefovir concentration range of 0.1 to 25 ug/mL. The volume of distribution at steady-state following intravenous administration of 1.0 or 3.0 mg/kg/day is 392 +/- 75 and 352 +/- 9 mL/kg, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
Food does not affect the area under the concentration-time curve (AUC) of adefovir.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 805
For more Absorption, Distribution and Excretion (Complete) data for Adefovir (10 total), please visit the HSDB record page.
9-(2-Phosphonylmethoxyethyl)adenine (PMEA) was the only metabolite formed after oral administration of bis-POM PMEA. Three metabolites were detected after oral administration of either bis-(phenyl) PMEA or bis-(o-ethoxyphenyl) PMEA to rats: PMEA, the corresponding monoester, and 2-adenylacetic acid. The major metabolite of bis-(phenyl) PMEA was 2-adenylacetic acid following oral administration. 2-Adenylacetic acid appears to have been formed from the intact prodrugs by a P450 mediated oxidation of the ethyl side chain.
PMID:9172955 Shaw JP et al; Drug Metab Dispos 25 (3): 362-6 (1997)
Following oral administration, adefovir dipivoxil is converted to the active adefovir.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 805
PMEA is a known human metabolite of pradefovir.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Plasma adefovir concentrations declined in a biexponential manner with a terminal elimination half-life of 7.48 +/- 1.65 hours.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
... Diphosphorylated ... PMEA has a relatively long intracellular half-life (16-18 hr) ...
PMID:1705039 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC51046 Balzarini J et al; Proc Natl Acad Sci (USA) 88 (4): 1499-503 (1991)
Adefovir dipivoxil is a prodrug of adefovir, an acyclic nucleotide analog antiviral agent. Following initial diester hydrolysis in vivo to form adefovir, the drug undergoes subsequent phosphorylation by cellular enzymes to form its active metabolite, adefovir diphosphate. Adefovir diphosphate inhibits hepatitis B virus (HBV) DNA polymerase (reverse transcriptase) by competing with the natural substrate deoxyadenosine triphosphate and by causing DNA chain termination after its incorporation into viral DNA. Adefovir diphosphate is a weak inhibitor of human DNA polymerases, including alpha- and gamma-polymerases.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 805
Adefovir is an acyclic nucleotide analog of adenosine monophosphate which is phosphorylated to the active metabolite adefovir diphosphate by cellular kinases. Adefovir diphosphate inhibits HBV DNA polymerase (reverse transcriptase) by competing with the natural substrate deoxyadenosine triphosphate and by causing DNA chain termination after its incorporation into viral DNA. The inhibition constant (Ki) for adefovir diphosphate for HBV DNA polymerase was 0.1 uM. Adefovir diphosphate is a weak inhibitor of human DNA polymerases alpha and gamma with Ki values of 1.18 uM and 0.97 uM, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for HEPSERA (adefovir dipivoxil) tablet (November 2012). Available from, as of November 14, 2012: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc
9-(2-Phosphonylmethoxyethyl)adenine (PMEA) is a potent and selective inhibitor of retrovirus (i.e., human immunodeficiency virus) replication in vitro and in vivo. Uptake of PMEA by human MT-4 cells and subsequent conversion to the mono- and diphosphorylated metabolites (PMEAp and PMEApp) are dose-dependent and occur proportionally with the initial extracellular PMEA concentrations. Adenylate kinase is unable to phosphorylate PMEA. However, 5-phosphoribosyl-1-pyrophosphate synthetase directly converts PMEA to PMEApp with a Km of 1.47 mM and a Vmax that is 150-fold lower than the Vmax for AMP. ATPase, 5'-phosphodiesterase, and nucleoside diphosphate kinase are able to dephosphorylate PMEApp to PMEAp, albeit to a much lower extent than the dephosphorylation of ATP. PMEApp has a relatively long intracellular half-life (16-18 hr) and has a much higher affinity for the human immunodeficiency virus-specified reverse transcriptase than for the cellular DNA polymerase alpha (Ki/Km: 0.01 and 0.60, respectively). PMEApp is at least as potent an inhibitor of human immunodeficiency virus reverse transcriptase as 2',3'-dideoxyadenosine 5'-triphosphate. Being an alternative substrate to dATP, PMEApp acts as a potent DNA chain terminator, and this may explain its anti-retrovirus activity.
PMID:1705039 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC51046 Balzarini J et al; Proc Natl Acad Sci (USA) 88 (4): 1499-503 (1991)
Global Sales Information

ABOUT THIS PAGE
81
PharmaCompass offers a list of Adefovir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Adefovir manufacturer or Adefovir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Adefovir manufacturer or Adefovir supplier.
PharmaCompass also assists you with knowing the Adefovir API Price utilized in the formulation of products. Adefovir API Price is not always fixed or binding as the Adefovir Price is obtained through a variety of data sources. The Adefovir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Adefovir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Adefovir, including repackagers and relabelers. The FDA regulates Adefovir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Adefovir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Adefovir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Adefovir supplier is an individual or a company that provides Adefovir active pharmaceutical ingredient (API) or Adefovir finished formulations upon request. The Adefovir suppliers may include Adefovir API manufacturers, exporters, distributors and traders.
click here to find a list of Adefovir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Adefovir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Adefovir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Adefovir GMP manufacturer or Adefovir GMP API supplier for your needs.
A Adefovir CoA (Certificate of Analysis) is a formal document that attests to Adefovir's compliance with Adefovir specifications and serves as a tool for batch-level quality control.
Adefovir CoA mostly includes findings from lab analyses of a specific batch. For each Adefovir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Adefovir may be tested according to a variety of international standards, such as European Pharmacopoeia (Adefovir EP), Adefovir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Adefovir USP).
