Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
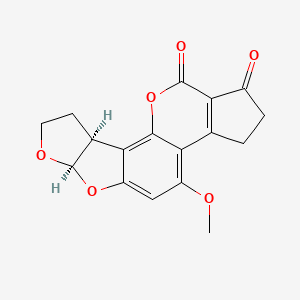

1. Aflatoxin B2 Alpha
2. Aflatoxin B2, (6ar-cis)-isomer
1. 7220-81-7
2. Dihydroaflatoxin B1
3. Dihydroaflatoxine B1
4. Aflatoxinb2
5. Aflatoxin-b(2)
6. Afb2
7. 7skr7s646p
8. (3s,7r)-11-methoxy-6,8,19-trioxapentacyclo[10.7.0.02,9.03,7.013,17]nonadeca-1,9,11,13(17)-tetraene-16,18-dione
9. (6ar,9as)-2,3,6a,8,9,9ahexahydro-4-methoxy-cyclopenta[c]furo[3',2':4,5]furo[2,3h][1]benzopyran-1,11-dione
10. (6ar,9as)-4-methoxy-2,3,6a,8,9,9a-hexahydrocyclopenta[c]furo[3',2':4,5]furo[2,3-h]chromene-1,11-dione
11. 2,3,6aalpha,8,9,9aalpha-hexahydro-4-methoxycyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzopyran-1,11-dione
12. 2,3,6aalpha,8,9,9aalpha-hexahydro-4-methoxycyclopenta[c]furo[3',2':4,5]furo[2,3-h][1]benzopyran-1,11-dione
13. 2,3,6aalpha,8,9,9aalpha-hexahydro-4-methoxycyclopenta[c]furo[3',2':4,5]furo[2,3-h]chromene-1,11-dione
14. Aflatoxin B2 0.5 Microg/ml In Acetonitrile
15. Ccris 13
16. Unii-7skr7s646p
17. Hsdb 3454
18. Cyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzopyran-1,11-dione, 2,3,6a,8,9,9a-hexahydro-4-methoxy-, (6ar,9as)-
19. Cyclopenta[c]furo[3',2':4,5]furo[2,3-h][1]benzopyran-1,11-dione, 2,3,6a,8,9,9a-hexahydro-4-methoxy-, (6ar,9as)-
20. Einecs 230-618-8
21. Brn 1355115
22. Aflatoxin B [mi]
23. Aflatoxin B2 [hsdb]
24. 5-19-10-00552 (beilstein Handbook Reference)
25. Dl-threo-ritalinic Acid Lactam
26. Schembl14424508
27. Chebi:48209
28. Aflatoxin B2, Reference Material
29. Dtxsid70222535
30. Zinc402720
31. Cyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzopyran-1,11-dione, 2,3,6a,8,9,9a-hexahydro-4-methoxy-, (6ar-cis)-
32. Mfcd00078140
33. Aflatoxin B2, From Aspergillus Flavus
34. Cyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzopyran-1,11-dione, 2,3,6aalpha,8,9,9aalpha-hexahydro-4-methoxy-
35. 4,5]furo[2,3-h][1]benzopyran-1,11-dione
36. 220a817
37. Q26840819
38. (6ar-cis)-2,3,6a,8,9,9a-hexahydro-4-methoxycyclopenta[c]furo[3',2'
39. Aflatoxin B2 Solution, 0.5 Mug/ml In Acetonitrile, Analytical Standard
40. Aflatoxin B2 Solution, 3 Mug/ml In Benzene:acetonitrile (98:2), Analytical Standard
41. Aflatoxin B2 Solution, 3.80 Mug/g In Acetonitrile, Erm(r) Certified Reference Material
42. (6ar-cis)-2,3,6a,8,9,9a-hexahydro-4-methoxycyclopenta[c]furo[3',2';4,5]furo[2,3-h][1]benzopyran-1,11-dione
43. 2,3,6aalpha,8,9,9aalpha-hexahydro-4- Methoxycyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzopyran- 1,11-dione
44. 2,3,6aalpha,8,9,9aalpha-hexahydro-4-methoxycyclopenta(c)furo(2',3':4,5)furo(2,3-h)chromene-1,11-dione
45. Aflatoxin B2 Solution, Certified Reference Material, 3 Mug/ml In Benzene:acetonitrile (98:2), Ampule Of 1 Ml
46. Aft
47. Cyclopenta(c)furo(3',2':4,5)furo(2,3-h)(1)benzopyran-1,11-dione, 2,3,6a-alpha,8,9,9a-alpha-hexahydro-4-methoxy-
| Molecular Weight | 314.29 g/mol |
|---|---|
| Molecular Formula | C17H14O6 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 314.07903816 g/mol |
| Monoisotopic Mass | 314.07903816 g/mol |
| Topological Polar Surface Area | 71.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 610 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Administration of (3)H-aflatoxin b2 to male rats gave levels of hepatic DNA & ribosomal (r)RNA aflatoxin adducts that were about 1% of those for rats given (3)H-aflatoxin b1. Levels of hepatic protein aflatoxin adducts were 35-70% as great for aflatoxin b2-treated rats as for aflatoxin b1-treated rats.
PMID:187331 Swenson DH et al; Cancer Res 37 (1): 172 (1977)
A study was conducted to determine aflatoxin levels in the tissues of broiler chickens that had been fed a diet containing 2057 micrograms aflatoxin B1 and 1323 micrograms aflatoxin B2/kg for 35 days. Results showed that aflatoxins were deposited in all tissues. The highest levels of aflatoxins were present in the gizzards, livers and kidneys. There was evidence that the high levels of aflatoxins B1 and B2 in the gizzards might have been caused by contamination by the gizzard contents during the slaughtering process. After feeding the aflatoxin-contaminated diet for 35 days, mean values for the combined aflatoxins were less than 3 micrograms/kg of tissue. Four days after withdrawal of the aflatoxin-contaminated ration, there were no detectable amounts of aflatoxins in any of the tissues. The results indicate that broiler chickens rapidly clear aflatoxins from their tissues once they are transferred to an aflatoxin-free diet.
Chen C et al; Food Chem Toxicol 22(6): p.447-451 (1984)
To evaluate the rate at which the four main aflatoxins (aflatoxins B1, B2, G1 and G2) are able to cross the luminal membrane of the rat small intestine, a study about intestinal absorption kinetics of these mycotoxins has been made. In situ results obtained showed that the absorption of aflatoxins in rat small intestine is a very fast process that follows first-order kinetics, with an absorption rate constant (ka) of 5.84 +/- 0.05 (aflatoxin B1), 4.06 +/- 0.09 (aflatoxin B2), 2.09 +/- 0.03 (aflatoxin G1) and 1.58 +/- 0.04 (aflatoxin G2) h-1, respectively.
Ramos A et al; Mycopathologia 134(1): p.27-30 (1996)
Yields Alfatoxin m2 in rat. From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. A-27
Metabolism of aflatoxin b2 by postmitochondrial supernatant fractions of duck, rat, mouse & human livers was studied in an in vitro system. Postmitochondrial supernatant from duck equivalent to 0.2 g whole liver metabolized 40-80% of the initial substrate in 30 min, compared to less than 6% for other species. Among several metabolites formed by duck liver, aflatoxin b1 was produced in amt equivalent to 2-8% of the initial substrate, & metabolites having chromatographic properties postulated for aflatoxicols 1 & 2 & aflatoxins m1 & m2 were also formed in small amounts. The greater susceptibility of duck liver to the toxicity of aflatoxin b2 may be attributable to its ability to form aflatoxin b1, which could be activated through further metabolism.
PMID:639051 Roebuck BD et al; Cancer Res 38 (4): 999 (1978)
Aflatoxin b2 admin iv to rats was rapidly metabolized to 7 groups of metabolites, 6 of which were excreted in the bile. Aflatoxin b2 was hydroxylated at the 2- & 4-positions. Bile from rats given aflatoxin b2 contained 2 glucuronides.
PMID:5034521 Dann RE et al; Res Commun Chem Pathol Pharmacol 3 (3): 667 (1972)
With the 4 principal aflatoxins tested, the order of inhibitory effect on RNA polymerase ii was: b1 greater than g1 greater than b1, g2.
Yu FL et al; Carcinogenesis (London) 3 (9): 1005 (1982)
Ability of aflatoxin b1, aflatoxin b2, & aflatoxin g1 to inhibit RNA polymerase activity & decr RNA content in rat hepatocyte nuclei was qualitatively similar to the carcinogenic & acute & subacute toxic actions of these compounds.
PMID:5120291 Edwards GS et al; Cancer Res 31 (12): 1943 (1971)
The interaction of aflatoxin B2 (AFB2) in vivo with rat liver nuclear macromolecules was examined in an attempt to correlate this binding with biological potency. The incorporation of [(3)H]AFB2 residues into rat liver histones and DNA was determined 2, 24 and 48 hr following administration of a single ip dose of 1 mg [(3)H]AFB2/kg bw. At each time point, histone H1 and the total histone fraction contained 5--30-fold more [(3)H]AFB2 moieties than did DNA on a weight basis. Analytical reversed-phase HPLC of the acid hydrolysis products resulting from AFB2 binding to DNA revealed that 85% of the radioactivity co-chromatographed with the major aflatoxin B1-DNA adduct, 2,3-dihydro-2-(N7-guanyl)-3-hydroxyaflatoxin B1. These studies revealed an apparent correlation between AFB2 derived binding to DNA in vivo in rats and its potency as a toxin and carcinogen in this species.
PMID:7326836 Groopman J et al; Carcinogenesis 2 (12): 1371-1373 (1981)
Aflatoxins produce singlet oxygen upon their exposure to UV (365-nm) light. Singlet oxygen in turn activates them to mutagens and DNA binding species. DNA binding and mutagenesis by aflatoxins were enhanced in D2O as compared to reactions in H2O, and a singlet oxygen scavenger inhibited mutagenesis. DNA photobinding of 3H-aflatoxin B1 increased in the presence of unlabeled aflatoxin B2, and the addition of aflatoxin B2 enhanced mutagenesis by aflatoxin B1 in a synergistic manner. These results are compatible with the notion that singlet oxygen, formed by one aflatoxin molecule, can readily activate another aflatoxin molecule. This may bear an environmental implication in that the weakly carcinogenic aflatoxin B2, which is often produced in nature together with aflatoxin B1, may be important in enhancing the activation of aflatoxin B1 by sunlight.
PMID:1900569 Stark AA, Liberman DF; Mutat Res 247 (1): 77-86 (1991)
ABOUT THIS PAGE
76
PharmaCompass offers a list of Aflatoxin B2 API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aflatoxin B2 manufacturer or Aflatoxin B2 supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aflatoxin B2 manufacturer or Aflatoxin B2 supplier.
PharmaCompass also assists you with knowing the Aflatoxin B2 API Price utilized in the formulation of products. Aflatoxin B2 API Price is not always fixed or binding as the Aflatoxin B2 Price is obtained through a variety of data sources. The Aflatoxin B2 Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aflatoxin B2 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aflatoxin B2, including repackagers and relabelers. The FDA regulates Aflatoxin B2 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aflatoxin B2 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Aflatoxin B2 supplier is an individual or a company that provides Aflatoxin B2 active pharmaceutical ingredient (API) or Aflatoxin B2 finished formulations upon request. The Aflatoxin B2 suppliers may include Aflatoxin B2 API manufacturers, exporters, distributors and traders.
Aflatoxin B2 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aflatoxin B2 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aflatoxin B2 GMP manufacturer or Aflatoxin B2 GMP API supplier for your needs.
A Aflatoxin B2 CoA (Certificate of Analysis) is a formal document that attests to Aflatoxin B2's compliance with Aflatoxin B2 specifications and serves as a tool for batch-level quality control.
Aflatoxin B2 CoA mostly includes findings from lab analyses of a specific batch. For each Aflatoxin B2 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aflatoxin B2 may be tested according to a variety of international standards, such as European Pharmacopoeia (Aflatoxin B2 EP), Aflatoxin B2 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aflatoxin B2 USP).
