


1. Ago 178
2. Ago-178
3. Ago178
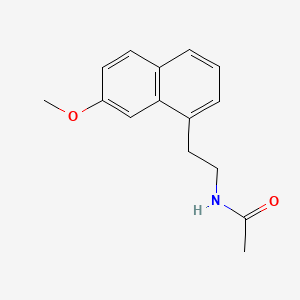
4. N-(2-(7-methoxy-1-naphthyl)ethyl)acetamide
5. S 20098
6. S-20098
7. S20098
8. Thymanax
9. Valdoxan
1. 138112-76-2
2. Thymanax
3. Valdoxan
4. N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide
5. N-(2-(7-methoxynaphthalen-1-yl)ethyl)acetamide
6. S20098
7. S-20098
8. Agomelatine [inn]
9. Melitor
10. Ago-178
11. S 20098
12. Acetamide, N-[2-(7-methoxy-1-naphthalenyl)ethyl]-
13. N-(2-(7-methoxy-1-naphthyl)ethyl)acetamide
14. N-(2-(7-methoxynaphth-1-yl)ethyl)acetamide
15. N-(2-(7-methoxy-1-naphthalenyl)ethyl)acetamide
16. Chembl10878
17. 137r1n49ad
18. N-[2-(7-methoxy-1-naphthalenyl)ethyl]acetamide
19. Agomelatine (inn)
20. Ncgc00253646-01
21. N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
22. Acetamide, N-(2-(7-methoxy-1-naphthalenyl)ethyl)-
23. N-[2-(7-methoxy-naphthalen-1-yl)-ethyl]-acetamide
24. Ago 178
25. Valdoxan (tn)
26. Unii-137r1n49ad
27. Ago178
28. N-[2-(7-methoxynaphth-1-yl)ethyl]acetamide
29. Sr-01000944938
30. Agomelatine [inn:ban]
31. Ago178c
32. Agomelatine-[d4]
33. Awy
34. Agomelatine- Bio-x
35. Mfcd00916659
36. Agomelatine [mi]
37. Agomelatine [mart.]
38. Dsstox_cid_31431
39. Dsstox_rid_97317
40. Dsstox_gsid_57642
41. Agomelatine [who-dd]
42. Gtpl198
43. Mls006011913
44. Schembl114476
45. Agomelatine [ema Epar]
46. Zinc5608
47. Dtxsid3057642
48. Agomelatine, >=98% (hplc)
49. Chebi:134990
50. Bcpp000421
51. Hms3604n09
52. Hms3648g18
53. Hms3654b07
54. Hms3884a07
55. Bcp02084
56. Tox21_113772
57. Bbl029084
58. Bdbm50035179
59. Pdsp1_001305
60. Pdsp1_001784
61. Pdsp2_001289
62. Pdsp2_001767
63. S1243
64. Stl237728
65. Akos005145681
66. Ac-3395
67. Bcp9000250
68. Ccg-221950
69. Cs-0740
70. Db06594
71. Ks-1247
72. Sb19508
73. Ncgc00253646-10
74. Ba167079
75. Hy-17038
76. Smr002530056
77. Am20090763
78. B2262
79. Cas-138112-76-2
80. Ft-0657383
81. Sw219177-1
82. A19445
83. D02578
84. Ab01274769-01
85. Ab01274769_02
86. 112a762
87. L000528
88. Q395229
89. Q-102507
90. Sr-01000944938-1
91. Sr-01000944938-3
92. F0001-2383
93. (4-chlorophenyl)-[(9h-fluoren-9-ylmethoxycarbonylamino)]-aceticacid
94. N-[2-(7-methoxy-naphthalen-1-yl)-ethyl]-acetamide(agomelatine)
| Molecular Weight | 243.30 g/mol |
|---|---|
| Molecular Formula | C15H17NO2 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 243.125928785 g/mol |
| Monoisotopic Mass | 243.125928785 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 280 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Agomelatine is indicated to treat major depressive episodes in adults.
Treatment of major depressive episodes in adults.
Treatment of major depressive episodes in adults.
Treatment of major depressive episodes
Agomelatine resynchronises circadian rhythms in animal models of delayed sleep phase syndrome and other circadian rhythm disruptions. It increases noradrenaline and dopamine release specifically in the frontal cortex and has no influence on the extracellular levels of serotonin. Agomelatine has shown an antidepressant-like effect in animal depression models, (learned helplessness test, despair test, and chronic mild stress) circadian rhythm desynchronisation, and in stress and anxiety models. In humans, agomelatine has positive phase shifting properties; it induces a phase advance of sleep, body temperature decline and melatonin onset. Controlled studies in humans have shown that agomelatine is as effective as the SSRI antidepressants paroxetine and sertraline in the treatment of major depression
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N06AX22
N06AX22
N06AX22
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AX - Other antidepressants
N06AX22 - Agomelatine
Absorption
Bioavailability is less than 5%.
Hepatic (90% CYP1A2 and 10% CYP2C9).
<2 hours
The novel antidepressant agent, agomelatine, behaves as an agonist at melatonin receptors (MT1 and MT2) and as an antagonist at serotonin (5-HT)(2C) receptors.

LOOKING FOR A SUPPLIER?