Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
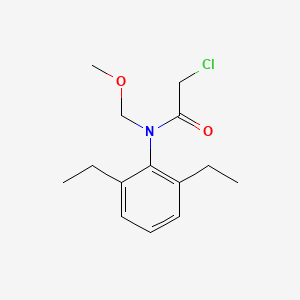

1. 2-chloro-n-(2,6-diethylphenyl)-n-(methoxymethyl)acetamide
2. Metachlor
1. 15972-60-8
2. Lasagrin
3. Metachlor
4. Methachlor
5. Alanex
6. 2-chloro-n-(2,6-diethylphenyl)-n-(methoxymethyl)acetamide
7. Alachlore
8. Alochlor
9. Pillarzo
10. Lasso
11. Chimiclor
12. Alanox
13. Lazo
14. Lasso Micro-tech
15. Alatox 480
16. 2-chloro-2',6'-diethyl-n-(methoxymethyl)acetanilide
17. Alazine
18. Acetamide, 2-chloro-n-(2,6-diethylphenyl)-n-(methoxymethyl)-
19. Alachlor Technical
20. Alachlor [ansi:bsi:iso]
21. Cp 50144
22. 2-chloro-n-(2,6-diethyl)phenyl-n-methoxymethylacetamide
23. Alachlor Technical (90% Or More)
24. N-(methoxymethyl)-2,6-diethylchloroacetanilide
25. Alpha-chloro-2',6'-diethyl-n-methoxymethylacetanilide
26. Chloressigsaeure-n-(methoxymethyl)-2,6-diaethylanilid
27. 24s2s61pxl
28. Acetanilide, 2-chloro-2',6'-diethyl-n-(methoxymethyl)-
29. Alapaz
30. Chebi:2533
31. Dsstox_cid_2265
32. Dsstox_rid_76532
33. Dsstox_gsid_22265
34. Caswell No. 011
35. Satochlor
36. Nitala
37. 2-chloro-n-(2,6-diethylphenyl)-n-[(methyloxy)methyl]acetamide
38. Alachlore [iso-french]
39. Alachlor [iso]
40. Cas-15972-60-8
41. Ccris 3155
42. Hsdb 1014
43. Einecs 240-110-8
44. Epa Pesticide Chemical Code 090501
45. Brn 2944476
46. Unii-24s2s61pxl
47. Ralchlor
48. Alagan
49. Ai3-51506
50. Alachlor-atrazine
51. Alachlor (pesticides Mixture)
52. Spectrum_001859
53. Alachlor [hsdb]
54. Specplus_000470
55. Chloressigsaeure-n-(methoxymethyl)-2,6-diaethylanilid [german]
56. Alachlor [mi]
57. Spectrum2_001853
58. Spectrum3_000835
59. Spectrum4_000675
60. Spectrum5_001984
61. Bidd:pxr0027
62. Schembl15501
63. Bspbio_002389
64. Kbiogr_001109
65. Kbioss_002376
66. Spectrum330043
67. 2-chloro-2',6'-diethyl-n-methoxymethylacetanilide
68. Mls002695955
69. Bidd:er0383
70. Divk1c_006566
71. Phenmetrazine-d5 Hydrochloride
72. Spbio_001666
73. 2',6'-diethyl-n-(methoxymethyl)-2-chloroacetanilide
74. Chembl1414154
75. Dtxsid1022265
76. Kbio1_001510
77. Kbio2_002372
78. Kbio2_004940
79. Kbio2_007508
80. Kbio3_001889
81. Xcsgpavhzfqhge-uhfffaoysa-
82. Alachlor 10 Microg/ml In Acetone
83. Hms3091a18
84. Zinc900557
85. Tox21_202205
86. Tox21_300713
87. Alachlor 100 Microg/ml In Methanol
88. Ccg-39391
89. Alachlor 1000 Microg/ml In Acetone
90. Akos015889908
91. Alachlor 5000 Microg/ml In Methanol
92. Alachlor 100 Microg/ml In Ethylacetate
93. Ncgc00090758-01
94. Ncgc00090758-02
95. Ncgc00090758-03
96. Ncgc00090758-04
97. Ncgc00090758-05
98. Ncgc00090758-06
99. Ncgc00090758-07
100. Ncgc00090758-08
101. Ncgc00090758-09
102. Ncgc00254619-01
103. Ncgc00259754-01
104. 1246817-91-3
105. Smr000777860
106. Alachlor, Pestanal(r), Analytical Standard
107. Ft-0657940
108. Ft-0661447
109. 972a608
110. Q421204
111. J-009637
112. 2-chloro-2', 6'-diethyl-n-(methoxymethyl)acetanilide
113. 2-chloro-2',6'-diethyl-n-(methoxymethyl) Acetanilide
114. 2-chloro-2',6'-diethyl-n-(methoxymethyl)-acetanilde
115. 2-chloro-2',6'-diethyl-n-(methoxymethyl)-acetanilide
116. Alachlor, Certified Reference Material, Tracecert(r)
117. Brd-k02548315-001-02-7
118. N-methoxymethyl-n-(2,6-diethylphenyl)chloroacetamide
119. N-(methoxymethyl)-2-chloro-n-(2,6-diethylphenyl)acetamide
120. 2-chloro-n-(methoxymethyl)-n-(2,6-diethyl-phenyl)-acetamide
121. 2-chloro-n-(2,6-diethylphenyl)-n-(methoxymethyl)acetamide, 9ci
122. Alachlor Solution, Certified Reference Material, 1000 Mug/ml In Methanol
| Molecular Weight | 269.77 g/mol |
|---|---|
| Molecular Formula | C14H20ClNO2 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 269.1182566 g/mol |
| Monoisotopic Mass | 269.1182566 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 248 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Herbicides
Pesticides used to destroy unwanted vegetation, especially various types of weeds, grasses (POACEAE), and woody plants. Some plants develop HERBICIDE RESISTANCE. (See all compounds classified as Herbicides.)
Three pharmacokinetic studies on Rhesus monkeys were performed: an intravenous route of administration study, dermal application of alachlor emulsifiable concentrate (EC), and a dermal application of alachlor micro-encapsulate formulation (Mcap). In all three studies, the levels of radioactivity were monitored in the blood for 7 days, and urine and feces for 9 to 14 days. The purpose of the intravenous study was to determine the pharmacokinetics of alachlor distribution and elimination. Two monkeys/sex/dose were given single doses of 0.24 or 2.4 mg/kg/day. Alachlor was rapidly distributed in the blood (whole, plasma, and red blood cells) within the first 15 minutes, and rapidly eliminated in urine primarily within the first 24 hours. Approximately 93.3% of the low dose and 99.6% of the high dose were eliminated in excreta during the 10-day study period. The majority of this elimination was via the urine (82.1% low dose, and 91.4%, high dose). In both the EC and the Mcap dermal studies, the formulations were tested undiluted and diluted (1:29 for EC and 1:17 for Mcap) with water, 2 monkeys/sex/formulation or dilution/EC or Mcap. The dosages (EC: 32 Fg/cm2 and 300 Fg/cm2; and Mcap 10.8 Fg/cm2 and 217 Fg/cm2) were applied to a 40 cm2 skin area and were left on the skin for 12 hours before removal. For the EC the rate of alachlor absorption was slow and reached a peak in the blood after 24 hours. The total dermal absorption in the low dose animals (32 Fg/cm2), estimated from excretion of radiolabel and retention of label in tissues, was 6-7% in males and 12-13% in females, uncorrected. However, calculation of the actual amount of test material absorbed through the skin was complicated by the fact that recovery of radiolabelled test material in this test group was poor, ranging from 21 to 77% of the nominal amount applied. Data were submitted demonstrating that up to 40% of the applied dose could apparently evaporate from skin (under conditions simulated in vitro) and that application error could result in application of up to 20% less than the nominal value. In the face of these uncertainties, values for excretion and absorption were calculated based upon the amount of radiolabel that was recovered. Using these correction factors, absorption was 10-24 % (low dose) in males and 16-20% (low dose) in females. For the EC, recovery of radiolabel was better in the high dose animals (300 Fg/cm2), and application of a correction factor had little effect. Absorption was 4-9% in males and 10-11% in females. It is also possible to estimate a percent dermal absorption by using a ratio of the corrected percent radiolabel excreted in urine after dermal application to the average percent radiolabel excreted in urine after intravenous administration, which is 87%. Using this ratio, the dermal absorption estimates for the low dose EC group were 9.2-24.8% for males and 16-21.8% for females. For the high dose EC group, the dermal absorption estimates were 4.7-8.9% for the males and 10.7-11.4% for the females. Thus, similar estimates of dermal absorption were obtained by either method of calculation. For the Mcap, the total dermal absorption for the low dose (10.8 Fg/cm2) ranged from 3-23% in males and 6-7% in females. For the high dose (217 Fg/cm2) the total dermal absorption ranged from 2-4% in males and 3-4% in females. Percent dermal absorptions were also estimated using the ratio specified in the discussion of the EC group. Using this ratio, the dermal absorption estimates for the low dose Mcap group were 3.2-23.4% for males and 6.7-7.1% for females. For the high dose Mcap group, the dermal absorption estimates were 2-3.8% in males, and 2.2-3.9% in females. Again, similar estimates of dermal absorption were obtained by either method of calculation.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.36 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
Another metabolism study was conducted on male and female Long Evans rats. This study consisted of seven groups of rats. Both oral dosing studies using corn oil as the vehicle and intravenous administration studies using propylene glycol as the vehicle were performed. ... The study is considered to be the definitive study for understanding how the rat metabolizes alachlor. Oral administration of alachlor was studied using female Long-Evans Crl:CD(LE)BR rats six to nine weeks of age in five dose groups. Groups 1, 2, and 3 each consisted of 33 rats. Each group received single oral doses of radiolabeled alachlor (uniformly labeled in the phenyl ring with 14-C, and enriched with 13-C at the C-2 carbon) at target doses of 7 (Group 1), 70 (Group 2), or 700 (Group 3) mg/kg. Group 4 consisted of 21 rats which received 15 consecutive daily doses of radiolabeled alachlor at 700 mg/kg/day. Group 5 consisted of 6 rats which received a single oral dose of radiolabeled alachlor at 700 mg/kg for the purpose of obtaining plasma samples at 2, 4, and 6 hours post-dosing. Long Evans rats (5/sex/dose) were used to study the disposition and metabolism of alachlor following intravenous administration at 7 (Group 6) or 70 (Group 7) mg/kg. In the oral studies, absorption at the 7 or 70 mg/kg dose levels was essentially complete, with a slight decrease in absorption at the 700 mg/kg dose level. Repeated oral dosing at 700 mg/kg had no significant effect on absorption. Residual radioactivity did not exceed 5% of the administered dose at any of the dose levels in this study. On a ug/g basis, the residual radioactivity in the non-glandular stomach was higher than in the glandular stomach except at 4 hours post-dose at the 700 mg/kg dose level. Decreasing the dose decreased the percentage of the dose in the non-glandular stomach but not in the glandular stomach. Nasal turbinates showed a secondary peak of radioactivity at 8 hours post-dose at the 700 or 70 mg/kg dose levels in contrast to other tissues. Excretion of alachlor derived radioactivity was approximately equivalent between urine and feces, with between 30-47% excreted in urine and 41-45% excreted in feces at single oral doses of 7, 70, or 700 mg/kg. Intravenous dosing at 7 or 70 mg/kg resulted in a similar excretion profile. Repeated oral dosing at 700 mg/kg resulted in a slight increase in fecal excretion of radioactivity...
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.20-21 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
In this study, male and female CD-1 mice (10/sex) received a single oral dose of radiolabeled alachlor in corn oil (890 mg/kg for male mice, 819 mg/kg for female mice). Urine and feces were collected daily for up to 7 days post-dose for analysis of excreted radioactivity and for identification of metabolites. In urine, 18.4+/-3.9% and 23.6+/-4.1% of the dose was excreted in male and female mice, respectively. In feces, 66.5+/-6.9% and 53.6+/-3.6% of the dose was excreted in male and female mice, respectively. Total recovery of radioactivity was 85.5+/-3.7% for male mice, and 79.4+/2.7% for female mice. (The low recoveries may be due to the fact that the mice were housed in pairs in units larger than those normally used for a mouse.) Analysis of blood at seven days post-dose showed 0.095+/-0.016% of the dose in males, and 0.075+/-0.017% of the dose in females. ... The data in this study show that in contrast to the rat, feces is the major route of excretion for alachlor derived radioactivity in CD-1 mice. The high percentage of fecal excretion could be the result of poor absorption of test chemical or extensive biliary excretion in the mouse.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.21-22 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
...The in vivo percutaneous absorption of alachlor in rhesus monkeys was 17.3 +/- 3.3, 15.3 +/- 3.9, and 21.4 +/- 14.2% for 24-hr skin exposure to Lasso formulation diluted 1:20, 1:40, and 1:80, respectively. In vivo, there was no support for increased alachlor skin absorption with water dilution, as previously reported for in vitro absorption. The average in vivo absorption of 18% applied dose over 24 hr (0.75%/h) was similar to the maximum in vitro rate of 0.8%/hr using human skin and human plasma as receptor fluid. Dose accountability in vivo was 80.6-95.2%. (14-14C)Alachlor in Lasso diluted 1:20 with water was placed on rhesus monkeys at concentrations of 23 micrograms/10 microliters/cm2. Skin decontamination at 0 h with soap and water (50% Ivory liquid 1:1 v/v with water) removed 73 +/- 15.8% (n = 4) of the applied dose with the first wash; this increased to a total of 82.3 +/- 14.8% with two additional washes. Decontamination after 1 h removed 87.5 +/- 12.4% with three successive washes. After 3 h decontamination ability decreased, and after 24 h only 51.9 +/- 12.2% could be recovered with three successive washes. Using water only, at 0 h 36.6 +/- 12.3% alachlor was removed with the first wash and the total increased to 56.0 +/- 14.0% with two additional washes. At 24 h the total amount decreased to 28.7 +/- 12.2% for three successive washes. Alachlor as Lasso in field-use rate (11 micrograms/cm2) and undiluted (217 and 300 micrograms/cm2) proportions were left on rhesus monkey skin for 12 h and decontaminated with soap and water (10% Ivory liquid v/v with water). Continual successive washes (6-8 in sequence) recovered 80-90% of the skin-applied alachlor. These results suggest that simple washing with soap and water is appropriate for removing some chemicals from skin. Decontamination with only water was less effective than with soap and water.
Wester RC et al; J Toxicol Environ Health 36 (1): 1-12 (1992).
The elimination of the product in both male and female rats is approximately equally distributed between the urine and feces. Nearly 90% of the administered dose is eliminated in 10 days. ... Radioactivity in rat tissues was concentrated in the highly perfused organs such as spleen, liver, kidney, and heart. Additional relatively high levels of radioactivity were found in the eyes, brain, stomach, and ovaries.
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1342
Another metabolism study was conducted on male and female Long Evans rats. This study consisted of seven groups of rats. Both oral dosing studies using corn oil as the vehicle and intravenous administration studies using propylene glycol as the vehicle were performed. ... The study is considered to be the definitive study for understanding how the rat metabolizes alachlor. Oral administration of alachlor was studied using female Long-Evans Crl:CD(LE)BR rats six to nine weeks of age in five dose groups. Groups 1, 2, and 3 each consisted of 33 rats. Each group received single oral doses of radiolabeled alachlor (uniformly labeled in the phenyl ring with 14-C, and enriched with 13-C at the C-2 carbon) at target doses of 7 (Group 1), 70 (Group 2), or 700 (Group 3) mg/kg. Group 4 consisted of 21 rats which received 15 consecutive daily doses of radiolabeled alachlor at 700 mg/kg/day. Group 5 consisted of 6 rats which received a single oral dose of radiolabeled alachlor at 700 mg/kg for the purpose of obtaining plasma samples at 2, 4, and 6 hours post-dosing. Long Evans rats (5/sex/dose) were used to study the disposition and metabolism of alachlor following intravenous administration at 7 (Group 6) or 70 (Group 7) mg/kg. ... In urine, the sec- amide hydroxymethyl sulfone metabolite (metabolite F5) of alachlor was the predominant urinary metabolite after oral and intravenous administration, ranging from 2.1-7.4% of the dose. Repeated oral dosing resulted in the appearance of several additional metabolites, but it is not known whether these additional metabolites are unique to repeated oral administration of alachlor. In feces, the tert-amide mercapturic acid and the disulfide appeared to be the major metabolites after single oral doses of alachlor. Increasing the dose appeared to increase the percentage of these 2 metabolites in feces.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.20-21 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
In this study, male and female CD-1 mice (10/sex) received a single oral dose of radiolabeled alachlor in corn oil (890 mg/kg for male mice, 819 mg/kg for female mice). Urine and feces were collected daily for up to 7 days post-dose for analysis of excreted radioactivity and for identification of metabolites. ... Pooled urine and fecal samples representing the 0-48 hour collection time for urine and the 0-96 hour collection time for feces, were analyzed for metabolites of alachlor in male and female CD-1 mice. In feces, at least 10 metabolites were isolated: ... /tert-amide mercapturic acid, disulfide conjugate, sec-amide mercapturic acid, tert-amide thioacetic acid, tert-amide hydroxy sulfone, tert-amide dihydroxysulfone, benzyl glucuronide and tert-amide cysteine conjugate +NCH20-glucuronide. Seven urinary metabolites were characterized in the mouse: tert-amide cysteine conjugate, NCH2O glucuronic acid, cysteine sulfoxide (proposed), sec-amide dihydroxysulfone, sec-amide hydroxy sulfoxide, sec-amide hydroxy sulfone and para-amino sulfate./ While metabolism of alachlor utilizes the same metabolic pathways in mice as in rats, there are quantitative differences between mice and rats in the metabolite profile present. Mouse feces were found to contain greater amounts of mercapturic acid conjugate and lesser amount of disulfide conjugate than in rat feces. The number of urinary metabolites observed in mouse urine was greater than in rat urine. Mouse urine was found to contain greater amounts of glucuronic acid conjugates and cysteine conjugates than the rat, but a lesser amount of phenolic (hydroxylated) metabolites.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.21-22 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
From a metabolism study in Rhesus monkeys, five urinary metabolites were identified after intravenous injection. One of these metabolites, (also found in rat and mouse urine, N-[2-ethyl-6-(1-hydroxyethyl)-phenyl]-N-(methoxymethyl)-2(methylsulfonyl)acetamide), tested positive in the Ames test with Salmonella typhimurium, with and without activation. This metabolite was an HEEA metabolite not previously identified in the monkey. Of the metabolites found in the above two metabolism studies, only two urinary metabolites were common to both the rat and monkey (secondary and tertiary mercapturic acid conjugates). Side chain hydroxylation and sulfate conjugation metabolites were not found in monkey urine as they were in rats.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.20 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
In the rat, alachlor is metabolized and eliminated as conjugates of mercapturic acid, glucuronic acid, and sulfate in the urine and feces
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1342
For more Metabolism/Metabolites (Complete) data for ALACHLOR (6 total), please visit the HSDB record page.
Alachlor has known human metabolites that include 2,6-Diethyl-N-(methoxymethyl)aniline, 2-Chloro-N-(2,6-diethylphenyl)acetamide, and S-(alachlor)glutathione.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Metabolism studies in Sprague Dawley rats found that an oral dose of 7 or 700 mg/kg of alachlor was mainly eliminated in urine and feces, and that 89% of the dose was eliminated in 10 days (minimal alachlor was found in the expired CO2). The elimination was considered to be biphasic; the initial rapid phase had a half life of 0.2 to 10.6 hours, which then slowed to a half life of 5 to 16 days.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.20 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
In this study, male and female CD-1 mice (10/sex) received a single oral dose of radiolabeled alachlor in corn oil (890 mg/kg for male mice, 819 mg/kg for female mice). Urine and feces were collected daily for up to 7 days post-dose ... Half life for urinary elimination was reported as 0.88+/-0.11 days in males, and 1.18+/-0.16 days in females. Half-life for fecal elimination was reported as 0.90+/-0.06 days in males, and 1.11+/-0.05 days in females.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.21-22 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
Mode of Action /in target species/: Chloroacetamides are known to inhibit biosynthesis of fatty acids, lipids, protein, isoprenoids, flavonoids, and gibberellins.
USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Alachlor. p.2 (December 1998). Available from, as of January 30, 2007: https://www.epa.gov/pesticides/reregistration/status.htm
The mechanism of thyroid hyperplasia and carcinogenicity of alachlor in the rat is based on the induction of T4/T3 glucuronic acid transferase (UDPGT) in the liver. Due to the enhanced glucuronidation of T4 and T3 and subsequent biliary excretion of the conjugates, the plasma concentrations of T4 and T3 are decreased. To compensate for this change, the pituitary produces more thyroid-stimulating hormone (TSH) to enhance T4 production by the thyroid. Continuous stimulation of the thyroid by increased plasma TSH levels leads to hyperplasia of the follicular epithelium and subsequently to tumor formation. The occurrance of thyroid follicular tumors following induction of hepatic UDPGT is a well-known phenomena in rats and is generally considered to be of little to no relevance for human risk assessment. Alachlor does not bind to human thyroid hormone receptor, human thyroid binding globulin or human transthyretin.
Alachlor: Evaluation of the Potential for Endocrine Disruption, 22 p. (December 20, 2002). Supporting and Related Materials in Alachlor Docket (EPA-HQ-OPP-2005-0050-0074). Accessed through www.regulations.gov.
Acetochlor, Alachlor and Butachlor may be grouped together based on a common end-point (nasal turbinate tumors in rats) and a known mechanism of toxicity for this endpoint. All three compounds produce tumors of the nasal olfactory epithelium in rats by way of a non-linear, non-genotoxic mode of action that includes cytotoxicity of the olfactory epithelium, followed by regenerative cell proliferation of the nasal epithelium that can then lead to neoplasia if cytotoxicity and proliferation are sustained. Acetochlor, Alachlor and Butachlor may also be grouped together based on an common end-point and a known mechanism of toxicity (UDPGT induction). All three compounds produce tumors of the thyroid follicular cells in rats by way of a non-genotoxic mode of action that includes UDPGT induction, increased TSH, alterations in T3/T4 hormone production and thyroid hyperplasia. The FIFRA Science Advisory Panel noted in /1997/, additionally, that even though the evidence illustrated that a common mechanism could be used to group certain chemicals for the development of thyroid tumors, it was recommended that this endpoint not be used in combining margins of exposure because the toxic effects were noted at doses above the Maximum Tolerated Dose (MTD).
US EPA; Cumulative Risk from Chloroacetanilide Pesticides. U.S. Environmental Protection Agency Office of Pesticide Programs Health Effects Division, 74 p (March 8, 2006).
...there is ample evidence ...that the development of nasal olfactory epithelium tumors in rats dosed with chloroacetanilides involves the following sequence of steps,: 1) Acetochlor conjugates with glutathione (GSH) and is excreted in the bile. 2) The conjugate is biotransformed to a series of sulfur-containing products. Enterohepatic circulation of these products creates a pool of metabolites that are delivered to the nose. 3) Biotransformation to tissue-reactive and toxic metabolites. Metabolism by nasal enzymes, results in formation of a benzoquinoneimine, an electrophile and redox-active molecule. 4) Binding of toxic metabolite to cellular proteins plus possible generation of oxidative stress . 5) Cytotoxicity 6) Regenerative cell proliferation. 6) Sustained cytotoxicity and cell proliferation that results in neoplasia. The following three events are considered key events for formation of nasal olfactory epithelium tumors by the proposed non-linear, non genotoxic mode of action (MOA): Quinone imine- formation (protein binding) goes to cytotoxicity goes to cell proliferation.
US EPA; Cumulative Risk from Chloroacetanilide Pesticides. U.S. Environmental Protection Agency Office of Pesticide Programs Health Effects Division, 74 p (March 8, 2006).
For more Mechanism of Action (Complete) data for ALACHLOR (9 total), please visit the HSDB record page.
ABOUT THIS PAGE
82
PharmaCompass offers a list of Alachlor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Alachlor manufacturer or Alachlor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Alachlor manufacturer or Alachlor supplier.
PharmaCompass also assists you with knowing the Alachlor API Price utilized in the formulation of products. Alachlor API Price is not always fixed or binding as the Alachlor Price is obtained through a variety of data sources. The Alachlor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Alachlor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Alachlor, including repackagers and relabelers. The FDA regulates Alachlor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Alachlor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Alachlor supplier is an individual or a company that provides Alachlor active pharmaceutical ingredient (API) or Alachlor finished formulations upon request. The Alachlor suppliers may include Alachlor API manufacturers, exporters, distributors and traders.
Alachlor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Alachlor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Alachlor GMP manufacturer or Alachlor GMP API supplier for your needs.
A Alachlor CoA (Certificate of Analysis) is a formal document that attests to Alachlor's compliance with Alachlor specifications and serves as a tool for batch-level quality control.
Alachlor CoA mostly includes findings from lab analyses of a specific batch. For each Alachlor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Alachlor may be tested according to a variety of international standards, such as European Pharmacopoeia (Alachlor EP), Alachlor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Alachlor USP).
