
Synopsis
Synopsis
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
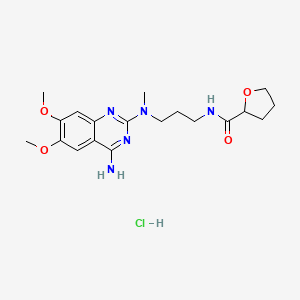

1. Alfetim
2. Alfusozine
3. Alfuzosin
4. Alphuzosine
5. Benestan
6. N-(3-((4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino)propyl)tetrahydro-2-furancarboxamide
7. Urion
8. Uroxatral
9. Xatral
1. 81403-68-1
2. Alfuzosin Hcl
3. Uroxatral
4. Urion
5. Xatral
6. Alfoten
7. Alfuzosin (hydrochloride)
8. Sl 77 499-10
9. Sl 77499-10
10. Alfuzosinhydrochloride
11. Sl-77499-10
12. 75046a1xtn
13. Alfuzosin Hydrochloride (uroxatral)
14. Alfetim
15. Sl-77-499-10
16. Dsstox_cid_25514
17. Dsstox_rid_80924
18. Dsstox_gsid_45514
19. N-(3-((4-amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino)propyl)tetrahydrofuran-2-carboxamide Hydrochloride
20. N-[3-[(4-amino-6,7-dimethoxyquinazolin-2-yl)-methylamino]propyl]oxolane-2-carboxamide;hydrochloride
21. Sl-7749910
22. 81403-68-1 (alfuzosin Hcl), 81403-80-7(alfuzosin).
23. Alfuzosin Hydrochloride [usan]
24. Xatral Retard
25. Xatral Od
26. Xatral Sr
27. Xatral Xl
28. Chebi:32286
29. Uroxatral (tn)
30. N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide, Monohydrochloride
31. Unii-75046a1xtn
32. N-(3-((4-amino-6,7-dimethoxyquinazolin-2-yl)
33. Sr-01000759345
34. Alfuzosin Hydrochloride (jan/usan)
35. Alfuzosin Hydrochloride [usan:usp]
36. Mfcd00879135
37. Sl-77499
38. Alfuzosin Hydrochloride;
39. Ncgc00016946-01
40. Sl77.0499-10
41. Cas-81403-68-1
42. Sl-77.0499-10
43. Alfuzosin Hydrochloride,(s)
44. Chembl1723
45. Alfuzosin-[d3] Hydrochloride
46. Schembl179910
47. Dtxsid3045514
48. Alfuzosin For Peak Identification
49. Hy-b0192a
50. Alfuzosin For System Suitability A
51. Bcpp000417
52. Hms1569a05
53. Alfuzosin Hydrochloride [mi]
54. Tox21_110701
55. Tox21_500224
56. Alfuzosin Hydrochloride [jan]
57. S1409
58. (methyl)amino)propyl)tetrahydrofuran-2
59. Akos015966770
60. Tox21_110701_1
61. Ac-1116
62. Alfuzosin Hydrochloride [mart.]
63. Bcp9000271
64. Ccg-213373
65. Lp00224
66. Alfuzosin Hydrochloride [usp-rs]
67. Alfuzosin Hydrochloride [who-dd]
68. Ncgc00095152-09
69. Ncgc00260909-01
70. 2-furancarboxamide, N-(3-((4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino)propyl)tetrahydro-, Monohydrochloride (+-)-
71. As-14240
72. Alfuzosin Hydrochloride [orange Book]
73. Ft-0630890
74. Sw196913-4
75. Alfuzosin Hydrochloride [ep Monograph]
76. Alfuzosin Hydrochloride [usp Monograph]
77. D01692
78. T71347
79. Alfuzosin Hydrochloride, >=98% (hplc), Solid
80. 403a681
81. A840121
82. Sr-01000759345-5
83. Q27114856
84. Alfuzosin Hydrochloride, European Pharmacopoeia (ep) Reference Standard
85. Alfuzosin Hydrochloride, United States Pharmacopeia (usp) Reference Standard
86. Alfuzosin For Peak Identification, European Pharmacopoeia (ep) Reference Standard
87. Alfuzosin For System Suitability A, European Pharmacopoeia (ep) Reference Standard
88. Alfuzosin For System Suitability, European Pharmacopoeia (ep) Reference Standard
89. Alfuzosin Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
90. (+/-)-n-(3-((4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino)propyl)tetrahydro-2-furamide Monohydrochloride
91. 2-furancarboxamide, (+/-)-n-(3-((4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino)propyl)tetrahydro-, Monohydrochloride
92. 4-amino-6,7-dimethoxy-2-[methyl[3-[(tetrahydro-2-furoyl)amino]propyl]amino]quinazoline Hydrochloride
93. N-(3-((4-amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino)-propyl)tetrahydrofuran-2-carboxamide Hydrochloride
94. N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)(methyl)amino]propyl]tetrahydro-2-furancarboxamide Hydrochloride
95. N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide Hydrochloride
| Molecular Weight | 425.9 g/mol |
|---|---|
| Molecular Formula | C19H28ClN5O4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Exact Mass | 425.1829821 g/mol |
| Monoisotopic Mass | 425.1829821 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 511 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Alfuzosin hydrochloride |
| Drug Label | Each alfuzosin hydrochloride extended-release tablet contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white powder that melts at 231 to 233C. It is freely soluble in water, sparingly solub... |
| Active Ingredient | Alfuzosin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Wockhardt; Teva Pharms; Apotex; Aurobindo Pharma; Torrent Pharms; Invagen Pharms; Sun Pharma Global; Mylan |
| 2 of 4 | |
|---|---|
| Drug Name | Uroxatral |
| PubMed Health | Alfuzosin (By mouth) |
| Drug Classes | Benign Prostatic Hypertrophy Agent |
| Drug Label | Each UROXATRAL extended-release tablet contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white crystalline powder that melts at approximately 240C. It is freely soluble in water, sparingly so... |
| Active Ingredient | Alfuzosin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Covis Pharma Sarl |
| 3 of 4 | |
|---|---|
| Drug Name | Alfuzosin hydrochloride |
| Drug Label | Each alfuzosin hydrochloride extended-release tablet contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white powder that melts at 231 to 233C. It is freely soluble in water, sparingly solub... |
| Active Ingredient | Alfuzosin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Wockhardt; Teva Pharms; Apotex; Aurobindo Pharma; Torrent Pharms; Invagen Pharms; Sun Pharma Global; Mylan |
| 4 of 4 | |
|---|---|
| Drug Name | Uroxatral |
| PubMed Health | Alfuzosin (By mouth) |
| Drug Classes | Benign Prostatic Hypertrophy Agent |
| Drug Label | Each UROXATRAL extended-release tablet contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white crystalline powder that melts at approximately 240C. It is freely soluble in water, sparingly so... |
| Active Ingredient | Alfuzosin hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Covis Pharma Sarl |
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
Adrenergic alpha-1 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC ALPHA-1 RECEPTORS. (See all compounds classified as Adrenergic alpha-1 Receptor Antagonists.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?