
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
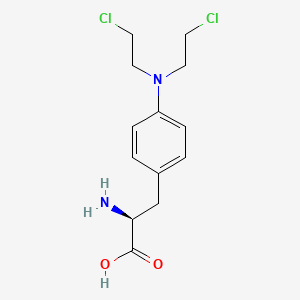

1. 4-(bis(2-chloroethyl)amino)phenylalanine
2. Alkeran
3. L-pam
4. Medphalan
5. Merphalan
6. Mustard, Phenylalanine
7. Phenylalanine Mustard
8. Sarcolysine
9. Sarkolysin
1. 148-82-3
2. Alkeran
3. Melfalan
4. L-pam
5. Phenylalanine Mustard
6. L-sarcolysine
7. L-phenylalanine Mustard
8. L-sarcolysin
9. P-l-sarcolysin
10. 4-[bis(2-chloroethyl)amino]-l-phenylalanine
11. Melphalanum
12. Melfalano
13. Phenylalanine Nitrogen Mustard
14. Levofalan
15. P-di-(2-chloroethyl)amino-l-phenylalanine
16. Sarcolysinum
17. Cb 3025
18. Nci-c04853
19. Levopholan
20. Levofolan
21. P-bis(beta-chloroethyl)aminophenylalanine
22. L-3-(p-(bis(2-chloroethyl)amino)phenyl)alanine
23. 3-(p-(bis(2-chloroethyl)amino)phenyl)-l-alanine
24. L-sarkolysin
25. P-n-bis(2-chloroethyl)amino-l-phenylalanine
26. 4-(bis(2-chloroethyl)amino)-l-phenylalanine
27. (2s)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoic Acid
28. 3-p-(di(2-chloroethyl)amino)-phenyl-l-alanine
29. Alanine Nitrogen Mustard
30. Cb-3025
31. Sk 15673
32. Sk-15673
33. Sarcolycin, L-
34. Nsc-8806
35. Evomela
36. L-phenylalanine, 4-(bis(2-chloroethyl)amino)-
37. Chebi:28876
38. P-n,n-bis(2-chloroethyl)amino-l-phenylalanine
39. Chembl852
40. Alkeran (tn)
41. Rcra Waste Number U150
42. Q41or9510p
43. Alanine, 3-(p-(bis(2-chloroethyl)amino)phenyl)-, L-
44. Alanine, 3-[p-[bis(2-chloroethyl)amino]phenyl]-, L-
45. Nsc-241286
46. 8057-25-8
47. Ncgc00090757-02
48. (2s)-2-azaniumyl-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoate
49. Dsstox_cid_804
50. L-phenylalanine, 4-[bis(2-chloroethyl)amino]-
51. Dsstox_rid_75797
52. Dsstox_gsid_20804
53. Melfalano [inn-spanish]
54. Melphalanum [inn-latin]
55. Phenylalanine, 4-[bis(2-chloroethyl)amino]-
56. (s)-2-amino-3-(4-(bis(2-chloroethyl)amino)phenyl)propanoic Acid
57. (2s)-2-amino-3-(4-[bis(2-chloroethyl)amino]phenyl)propanoic Acid
58. (s)-2-amino-3-{4-[bis-(2-chloro-ethyl)-amino]-phenyl}-propionic Acid
59. Nsc8806
60. Cas-148-82-3
61. Smr000058720
62. Ccris 374
63. Hsdb 3234
64. Einecs 205-726-3
65. Rcra Waste No. U150
66. Nsc 241286
67. Brn 2816456
68. L-3-(para-(bis(2-chloroethyl)amino)phenyl)alanine
69. Phelinun
70. Unii-q41or9510p
71. (2s)-2-amino-3-{4-[bis(2-chloroethyl)amino]phenyl}propanoic Acid
72. Nsc241286
73. Melphalan, Powder
74. Prestwick_1006
75. Melphalan [usan:usp:inn:ban:jan]
76. Spectrum_000397
77. Melphalan [inn]
78. Melphalan [jan]
79. Melphalan [mi]
80. 3025 C.b.
81. Melphalan [hsdb]
82. Melphalan [iarc]
83. Melphalan [usan]
84. Spectrum2_000074
85. Spectrum3_000684
86. Spectrum4_000882
87. Spectrum5_001601
88. Melphalan [vandf]
89. Dl-melphalan Hydrochloride
90. 3-(p-(bis(2-chloroethyl)amino)phenyl)alanine
91. Epitope Id:141802
92. Melphalan [mart.]
93. 4-[bis-(2-chloroethyl)amino]-l-phenylalanine
94. Melphalan [who-dd]
95. Schembl5872
96. Bspbio_002407
97. Kbiogr_001284
98. Kbioss_000877
99. 4-14-00-01689 (beilstein Handbook Reference)
100. Mls001333666
101. Mls002153368
102. Bidd:gt0044
103. Divk1c_000653
104. Spectrum1500382
105. Spbio_000287
106. Melphalan (jp17/usp/inn)
107. Gtpl7620
108. Zinc1673
109. Melphalan [orange Book]
110. Dtxsid6020804
111. Melphalan [ep Monograph]
112. Niosh/ay3360000
113. Hms502a15
114. Kbio1_000653
115. Kbio2_000877
116. Kbio2_003445
117. Kbio2_006013
118. Kbio3_001627
119. L-phenylalanine Mustard (l-pam)
120. Melphalan [usp Monograph]
121. Ninds_000653
122. Hms2090b09
123. Hms2091b16
124. Hms2235d21
125. Pharmakon1600-01500382
126. Tox21_111010
127. Tox21_202522
128. Bdbm50025837
129. Ccg-39704
130. Dl-442
131. Mfcd00057717
132. Nsc757098
133. S8266
134. Akos015895374
135. Tox21_111010_1
136. Cs-3120
137. Db01042
138. Nsc-757098
139. Idi1_000653
140. Smp2_000174
141. Ncgc00090757-01
142. Ncgc00090757-03
143. Ncgc00090757-04
144. Ncgc00090757-05
145. Ncgc00090757-06
146. Ncgc00260071-01
147. As-13314
148. Hy-17575
149. Sbi-0052787.p003
150. Ay33600000
151. 4-[bis(2-chloroethyl)-amino]-l-phenylalanine
152. 4-[bis(2-chloroethyl)-amino]-l-phenyl-alanine
153. 4-[bis(2-chloroethyl)amino]-(l)-phenylalanine
154. C07122
155. D00369
156. Ab00053282-07
157. Ab00053282-08
158. Ab00053282_09
159. 148m823
160. A808810
161. Alanine, 3-(p-(bis(2-chloroethyl)amino)phenyl)-
162. Sr-05000001667
163. Q2298283
164. Sr-05000001667-1
165. W-108096
166. Brd-k87827419-001-02-8
167. 2-amino-3-[4-bis(2-chloroethyl)amino]phenylpropanoic Acid
168. (2s)-2-azanyl-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoic Acid
169. 2-amino-3-{4-[bis-(2-chloro-ethyl)-amino]-phenyl}-propionic Acid
170. 2-amino-3-{4-[bis-(2-chloro-ethyl)-amino]-phenyl}-propionic Acid(l-pam)
171. 2-amino-3-{4-[bis-(2-chloro-ethyl)-amino]-phenyl}-propionic Acid (melphalan)
172. 2-amino-3-{4-[bis-(2-chloro-ethyl)-amino]-phenyl}-propionic Acid(melphalan)
| Molecular Weight | 305.20 g/mol |
|---|---|
| Molecular Formula | C13H18Cl2N2O2 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 304.0745332 g/mol |
| Monoisotopic Mass | 304.0745332 g/mol |
| Topological Polar Surface Area | 66.6 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 265 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Alkeran |
| PubMed Health | Melphalan (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | ALKERAN (melphalan), also known as L-phenylalanine mustard, phenylalanine mustard, L-PAM, or L-sarcolysin, is a phenylalanine derivative of nitrogen mustard. Melphalan is a bifunctional alkylating agent which is active against selective human neoplas... |
| Active Ingredient | Melphalan hydrochloride; Melphalan |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 2mg; eq 50mg base/vial |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 2 | |
|---|---|
| Drug Name | Alkeran |
| PubMed Health | Melphalan (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | ALKERAN (melphalan), also known as L-phenylalanine mustard, phenylalanine mustard, L-PAM, or L-sarcolysin, is a phenylalanine derivative of nitrogen mustard. Melphalan is a bifunctional alkylating agent which is active against selective human neoplas... |
| Active Ingredient | Melphalan hydrochloride; Melphalan |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 2mg; eq 50mg base/vial |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Antineoplastic Agents, Alkylating; Myeloablative Agonists
National Library of Medicine's Medical Subject Headings. Melphalan. Online file (MeSH, 2016). Available from, as of December 5, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Melphalan is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=MELPHALAN&Search=Search
Melphalan is used as a drug to treat cancer and other medical conditions, including ovarian cancer, malignant melanoma, multiple myeloma, breast cancer, advanced prostate cancer, testicular cancer, chronic myelogenous leukemia, osteogenic sarcoma, polycythemia vera, amyloidosis, and scleromyxedema (a rare skin disease). It is also used as an insect chemosterilant.
NTP; Report on Carcinogens, Fourteenth Edition (November, 2016); Available from, as of December 5, 2016: https://ntp.niehs.nih.gov/go/roc
Melphalan is used alone and as a component of various chemotherapeutic regimens in the treatment of myltiple myeloma.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1172
For more Therapeutic Uses (Complete) data for Melphalan (17 total), please visit the HSDB record page.
Although its safety during pregnancy has not been evaluated, melphalan is potentially teratogenic and should not be used during this period unless absolutely necessary.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 2015
Melphalan should be used cautiously in patients with severe renal insufficiency.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 2015
With prolonged use, sterility occurs with all alkylators; females appear more sensitive than males. /Alkalating agents, oral/
Knoben, J.E. and P.O. Anderson (eds.) Handbook of Clinical Drug Data. 6th ed. Bethesda, MD: Drug Intelligence Publications, Inc. 1988., p. 400
Arterial or venous thrombosis, or pulmonary embolism, sometimes fatal, has been reported in patients receiving melphalan by regional isolated perfusion.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1172
For more Drug Warnings (Complete) data for Melphalan (11 total), please visit the HSDB record page.
For the palliative treatment of multiple myeloma and for the palliation of non-resectable epithelial carcinoma of the ovary. Has also been used alone or as part of various chemotherapeutic regimens as an adjunct to surgery in the treatment of breast cancer, alone or in combination regimens for palliative treatment of locally recurrent or unresectable in-transit metastatic melanoma of the extremities, as well as for the treatment of amyloidosis with prednisone.
FDA Label
High-dose of Phelinun used alone or in combination with other cytotoxic medicinal products and/or total body irradiation is indicated in the treatment of:
- multiple myeloma,
- malignant lymphoma (Hodgkin, non-Hodgkin lymphoma),
- acute lymphoblastic and myeloblastic leukemia,
- childhood neuroblastoma,
- ovarian cancer ,
- mammary adenocarcinoma.
Phelinun in combination with other cytotoxic medicinal products is indicated as reduced intensity conditioning (RIC) treatment prior to allogeneic haematopoietic stem cell transplantation (allo-HSCT) in malignant haematological diseases in adults.
Phelinun in combination with other cytotoxic medicinal products is indicated as conditioning regimen prior to allogeneic haematopoietic stem cell transplantation in haematological diseases in the paediatric population as:
- Myeloablative conditioning (MAC) treatment in case of malignant haematological diseases
- RIC treatment in case of non-malignant haematological diseases.
Melphalan is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Alkylating agents are cell cycle-nonspecific. Alkylating agents work by three different mechanisms all of which achieve the same end result - disruption of DNA function and cell death.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
Myeloablative Agonists
Agents that destroy bone marrow activity. They are used to prepare patients for BONE MARROW TRANSPLANTATION or STEM CELL TRANSPLANTATION. (See all compounds classified as Myeloablative Agonists.)
L01AA03
L01AA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AA - Nitrogen mustard analogues
L01AA03 - Melphalan
Absorption
Incomplete, variable, 25-89% post oral dose
Route of Elimination
The 24-hour urinary excretion of parent drug in these patients was 10% 4.5%, suggesting that renal clearance is not a major route of elimination of parent drug.
Volume of Distribution
0.5 L/kg
Absorption melphalan from the GI tract is incomplete and extremely variable.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1172
After admin of (3)H-labeled melphalan to Walker carcinoma-bearing rats, radioactivity was found in liver, spleen, kidney, intestine and Walker carcinoma and to lesser extent in bone marrow, muscle, skin, testis and brain, but not in DNA fraction of any of these tissues. Approx 25% of admin radioactivity was excreted in urine during 1st 48 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V9 174 (1975)
We have studied the disposition and elimination of melphalan after intravenous administration in 9 patients with cancer. High-pressure liquid chromatography and (14)C-melphalan were used to assay drug concentration in plasma and urine. Composite plasma t1/2alpha was 7.7 +/- 3.3 and t1/2beta was 108 +/- 20.8 min for 8 of the patients. The mean 24-hr urinary excretion of melphalan was 13.0 +/- 5.4% of the administered dose. In 2 patients, 80% to 100% of the measured (14)C counts in plasma and urine samples at each study interval, up to 24 hr after drug administration, could be accounted for by the sum of parent compound, monohydroxy and dihydroxy products, and methanol nonextractable radioactivity (i.e., protein-bound activity). These data and evidence of rapid disappearance from plasma at 37 degrees /C/ in vitro suggest that spontaneous degradation, and not enzymatic metabolism, is the major determinant of the t1/2 of melphalan in vivo.
PMID:445964 Alberts DS et al; Clin Pharmacol Ther 26 (1): 73-80 (1979)
After iv admin of (14)C-labeled melphalan in female, weanling Yorkshire white pigs, tissue samples indicated bone and fat may be acting as depots for the drug. After 8 days, avg of 70% eliminated in urine. An avg of 4.02% of applied dose was absorbed after 14 days. Significant levels of radioactivity were also detected in skin surrounding site of application.
Skierkowski P et al; Report; Iss Oorder No PB-290436, 1978, 41
For more Absorption, Distribution and Excretion (Complete) data for Melphalan (12 total), please visit the HSDB record page.
Melphalan is not actively metabolised, it spontaneously degrades to mono and dihydroxy products.
... Most of administered dose is chemically altered and metabolites persist in the body.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 2015
We have studied the disposition and elimination of melphalan after intravenous administration in 9 patients with cancer. High-pressure liquid chromatography and (14)C-melphalan were used to assay drug concentration in plasma and urine. Composite plasma t1/2alpha was 7.7 +/- 3.3 and t1/2beta was 108 +/- 20.8 min for 8 of the patients. The mean 24-hr urinary excretion of melphalan was 13.0 +/- 5.4% of the administered dose. In 2 patients, 80% to 100% of the measured (14)C counts in plasma and urine samples at each study interval, up to 24 hr after drug administration, could be accounted for by the sum of parent compound, monohydroxy and dihydroxy products, and methanol nonextractable radioactivity (i.e., protein-bound activity). These data and evidence of rapid disappearance from plasma at 37 degrees /C/ in vitro suggest that spontaneous degradation, and not enzymatic metabolism, is the major determinant of the t1/2 of melphalan in vivo.
PMID:445964 Alberts DS et al; Clin Pharmacol Ther 26 (1): 73-80 (1979)
Melphalan is apparently eliminated from plasma by spontaneous hydrolysis, froming the monohydroxy and dihydroxy derivatives of the drug; no other metabolites have been identified in humans.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1172
1.5 (±0.83) hours
We have studied the disposition and elimination of melphalan after intravenous administration in 9 patients with cancer. High-pressure liquid chromatography and (14)C-melphalan were used to assay drug concentration in plasma and urine. Composite plasma t1/2alpha was 7.7 +/- 3.3 and t1/2beta was 108 +/- 20.8 min for 8 of the patients. The mean 24-hr urinary excretion of melphalan was 13.0 +/- 5.4% of the administered dose. In 2 patients, 80% to 100% of the measured 14C counts in plasma and urine samples at each study interval, up to 24 hr after drug administration, could be accounted for by the sum of parent compound, monohydroxy and dihydroxy products, and methanol nonextractable radioactivity (i.e., protein-bound activity). These data and evidence of rapid disappearance from plasma at 37 degrees /C/ in vitro suggest that spontaneous degradation, and not enzymatic metabolism, is the major determinant of the t1/2 of melphalan in vivo.
PMID:445964 Alberts DS et al; Clin Pharmacol Ther 26 (1): 73-80 (1979)
Intravenous admin (14)C-labeled melphalan in female, weanling Yorkshire white pigs indicated very slow elimination phase (half-life= 214 hr).
Skierkowski P et al; Report; Iss Oorder No PB-290436, 1978, 41
After iv admin in humans, the drug was distributed in total body water and disappeared with half-lives of approx 67 min and 160 hr. Up to 65% of label was recovered in urine over period of 7 days.
PMID:648565 Tattersall MH et al; Eur J Cancer 14 (5): 507-13 (1978)
The disappearance of L-PAM (intact drug) from the serum was biphasic after iv administration, with half-lives of 14 and 66 minutes for the alpha and beta phases, respectively.
PMID:597816 Furner RL et al; Cancer Treat Rep 61 (9): 1637-46 (1977)
For more Biological Half-Life (Complete) data for Melphalan (6 total), please visit the HSDB record page.
Melphalan attaches alkyl groups to the N-7 position of guanine and N-3 position of adenine, leading to the formation of monoadducts, and DNA fragmenting when repair enzymes attempt to correct the error. It can also cause DNA cross-linking from the N-7 position of one guanine to the N-7 position of another, preventing DNA strands from separating for synthesis or transcription. Finally, melphalan can induce a number of different mutations.
Melphalan, as an alkylating agent, interferes with DNA replication and transcription of RNA, and ultimately results in the disruption of nucleic acid function. Melphalan also possesses some immunosuppressive activity.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1172
Melphalan is a direct-acting alkylating agent that is carcinogenic via genotoxic mechanism.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V 100A P113

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
75
PharmaCompass offers a list of Melphalan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Melphalan manufacturer or Melphalan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Melphalan manufacturer or Melphalan supplier.
PharmaCompass also assists you with knowing the Melphalan API Price utilized in the formulation of products. Melphalan API Price is not always fixed or binding as the Melphalan Price is obtained through a variety of data sources. The Melphalan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Alkeran manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Alkeran, including repackagers and relabelers. The FDA regulates Alkeran manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Alkeran API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Alkeran manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Alkeran supplier is an individual or a company that provides Alkeran active pharmaceutical ingredient (API) or Alkeran finished formulations upon request. The Alkeran suppliers may include Alkeran API manufacturers, exporters, distributors and traders.
click here to find a list of Alkeran suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Alkeran DMF (Drug Master File) is a document detailing the whole manufacturing process of Alkeran active pharmaceutical ingredient (API) in detail. Different forms of Alkeran DMFs exist exist since differing nations have different regulations, such as Alkeran USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Alkeran DMF submitted to regulatory agencies in the US is known as a USDMF. Alkeran USDMF includes data on Alkeran's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Alkeran USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Alkeran suppliers with USDMF on PharmaCompass.
A Alkeran CEP of the European Pharmacopoeia monograph is often referred to as a Alkeran Certificate of Suitability (COS). The purpose of a Alkeran CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Alkeran EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Alkeran to their clients by showing that a Alkeran CEP has been issued for it. The manufacturer submits a Alkeran CEP (COS) as part of the market authorization procedure, and it takes on the role of a Alkeran CEP holder for the record. Additionally, the data presented in the Alkeran CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Alkeran DMF.
A Alkeran CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Alkeran CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Alkeran suppliers with CEP (COS) on PharmaCompass.
A Alkeran written confirmation (Alkeran WC) is an official document issued by a regulatory agency to a Alkeran manufacturer, verifying that the manufacturing facility of a Alkeran active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Alkeran APIs or Alkeran finished pharmaceutical products to another nation, regulatory agencies frequently require a Alkeran WC (written confirmation) as part of the regulatory process.
click here to find a list of Alkeran suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Alkeran as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Alkeran API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Alkeran as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Alkeran and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Alkeran NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Alkeran suppliers with NDC on PharmaCompass.
Alkeran Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Alkeran GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Alkeran GMP manufacturer or Alkeran GMP API supplier for your needs.
A Alkeran CoA (Certificate of Analysis) is a formal document that attests to Alkeran's compliance with Alkeran specifications and serves as a tool for batch-level quality control.
Alkeran CoA mostly includes findings from lab analyses of a specific batch. For each Alkeran CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Alkeran may be tested according to a variety of international standards, such as European Pharmacopoeia (Alkeran EP), Alkeran JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Alkeran USP).

