
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
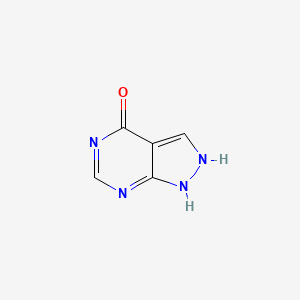

| Molecular Weight | 136.11 g/mol |
|---|---|
| Molecular Formula | C5H4N4O |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 136.03851076 g/mol |
| Monoisotopic Mass | 136.03851076 g/mol |
| Topological Polar Surface Area | 65.8 A^2 |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 275 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Allopurinol |
| PubMed Health | Allopurinol |
| Drug Classes | Antigout, Urinary Stone Agent |
| Drug Label | Allopurinol is known chemically as 1,5-Dihydro-4H-pyrazolo[3,4-d ]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Its solubility in water at 37C is 80 mg/dL and is greater in an alkaline solution. Allopurinol Table... |
| Active Ingredient | Allopurinol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Watson Labs; Vintage Pharms; Ipca Labs; Mutual Pharm; Apotex; Accord Hlthcare; Sun Pharm Inds; Northstar Hlthcare; Mylan |
| 2 of 10 | |
|---|---|
| Drug Name | Allopurinol sodium |
| PubMed Health | Allopurinol (Injection) |
| Drug Classes | Antigout, Urinary Stone Agent |
| Drug Label | ALOPRIM (allopurinol sodium) for Injection is the brand name for allopurinol, a xanthine oxidase inhibitor. ALOPRIM (allopurinol sodium) for Injection is a sterile solution for intravenous infusion only. It is available in vials as the sterile lyophi... |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 3 of 10 | |
|---|---|
| Drug Name | Aloprim |
| PubMed Health | Allopurinol |
| Drug Classes | Antigout, Urinary Stone Agent |
| Drug Label | ZYLOPRIM (allopurinol) has the following structural formula:ZYLOPRIM is known chemically as 1,5-dihydro-4H-pyrazolo [3,4-d]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Each scored white tablet contains 100 mg allo... |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 4 of 10 | |
|---|---|
| Drug Name | Lopurin |
| Active Ingredient | Allopurinol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 5 of 10 | |
|---|---|
| Drug Name | Zyloprim |
| Active Ingredient | Allopurinol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Prometheus Labs |
| 6 of 10 | |
|---|---|
| Drug Name | Allopurinol |
| PubMed Health | Allopurinol |
| Drug Classes | Antigout, Urinary Stone Agent |
| Drug Label | Allopurinol is known chemically as 1,5-Dihydro-4H-pyrazolo[3,4-d ]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Its solubility in water at 37C is 80 mg/dL and is greater in an alkaline solution. Allopurinol Table... |
| Active Ingredient | Allopurinol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Watson Labs; Vintage Pharms; Ipca Labs; Mutual Pharm; Apotex; Accord Hlthcare; Sun Pharm Inds; Northstar Hlthcare; Mylan |
| 7 of 10 | |
|---|---|
| Drug Name | Allopurinol sodium |
| PubMed Health | Allopurinol (Injection) |
| Drug Classes | Antigout, Urinary Stone Agent |
| Drug Label | ALOPRIM (allopurinol sodium) for Injection is the brand name for allopurinol, a xanthine oxidase inhibitor. ALOPRIM (allopurinol sodium) for Injection is a sterile solution for intravenous infusion only. It is available in vials as the sterile lyophi... |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 8 of 10 | |
|---|---|
| Drug Name | Aloprim |
| PubMed Health | Allopurinol |
| Drug Classes | Antigout, Urinary Stone Agent |
| Drug Label | ZYLOPRIM (allopurinol) has the following structural formula:ZYLOPRIM is known chemically as 1,5-dihydro-4H-pyrazolo [3,4-d]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Each scored white tablet contains 100 mg allo... |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 9 of 10 | |
|---|---|
| Drug Name | Lopurin |
| Active Ingredient | Allopurinol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Dr Reddys La |
| 10 of 10 | |
|---|---|
| Drug Name | Zyloprim |
| Active Ingredient | Allopurinol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Prometheus Labs |
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37104
Submission : 2023-01-20
Status : Active
Type : II

Certificate Number : CEP 2023-029 - Rev 00
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 576
Status : Valid

NDC Package Code : 49716-328
Start Marketing Date : 2023-10-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39568
Submission : 2024-03-05
Status : Active
Type : II

NDC Package Code : 61281-9750
Start Marketing Date : 2023-07-31
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.

Certificate Number : R1-CEP 1996-032 - Rev 05
Issue Date : 2019-08-09
Type : Chemical
Substance Number : 576
Status : Valid

Registration Number : 231MF10077
Registrant's Address : H-1106 BUDAPEST KERESZTURI UT 30-38 HUNGARY
Initial Date of Registration : 2019-03-29
Latest Date of Registration : --

Registrant Name : Masung LS Co., Ltd.
Registration Date : 2021-01-25
Registration Number : 20210125-209-J-511
Manufacturer Name : EGIS Pharmaceuticals Plc.
Manufacturer Address : Kereszturi ut 30-38., Budapest, 1106, Hungary
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Allopurinol Zentiva
Dosage Form : Tabl
Dosage Strength : 100mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Allopurinol Zentiva
Dosage Form : Tabl
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Allopurinol Zentiva
Dosage Form : Tabl
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
Regulatory Info :
Registration Country : Sweden
Brand Name : Zyloric
Dosage Form : TABLET
Dosage Strength : 100 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
Regulatory Info :
Registration Country : Sweden
Brand Name : Zyloric
Dosage Form : TABLET
Dosage Strength : 300 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 100 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 100 mg
Packaging : Box
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 300 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 300 mg
Packaging : Box
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Zyloric 300
Dosage Form : Tabl
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Allopurinol Zentiva
Dosage Form : Tabl
Dosage Strength : 100mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Allopurinol Zentiva
Dosage Form : Tabl
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Allopurinol Zentiva
Dosage Form : Tabl
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
Regulatory Info :
Registration Country : Sweden
Brand Name : Zyloric
Dosage Form : TABLET
Dosage Strength : 100 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
Regulatory Info :
Registration Country : Sweden
Brand Name : Zyloric
Dosage Form : TABLET
Dosage Strength : 300 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 100 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 100 mg
Packaging : Box
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 300 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
Regulatory Info :
Registration Country : Norway
Brand Name : Zyloric
Dosage Form : Antic-calc Tablet
Dosage Strength : 300 mg
Packaging : Box
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Zyloric 300
Dosage Form : Tabl
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : APO-ALLOPURINOL TABLETS
Dosage Form : TABLET
Dosage Strength : 100MG
Packaging : 100/1000
Approval Date :
Application Number : 402818
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : APO-ALLOPURINOL TABLETS
Dosage Form : TABLET
Dosage Strength : 300MG
Packaging : 100/500
Approval Date :
Application Number : 402796
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : APO-ALLOPURINOL
Dosage Form : TABLET
Dosage Strength : 300MG
Packaging : 100/500
Approval Date :
Application Number : 2402785
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : JAMP ALLOPURINOL
Dosage Form : TABLET
Dosage Strength : 100MG
Packaging : 100/1000
Approval Date :
Application Number : 2421593
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : JAMP ALLOPURINOL
Dosage Form : TABLET
Dosage Strength : 200MG
Packaging : 100/500
Approval Date :
Application Number : 2421607
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : JAMP ALLOPURINOL
Dosage Form : TABLET
Dosage Strength : 300MG
Packaging : 100/500
Approval Date :
Application Number : 2421615
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : MAR-ALLOPURINOL
Dosage Form : TABLET
Dosage Strength : 300MG
Packaging : 30/100/500/1000
Approval Date :
Application Number : 2396343
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Canada
Brand Name :
Dosage Form : TABLET
Dosage Strength : 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Canada
Brand Name :
Dosage Form : TABLET
Dosage Strength : 200MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : ALLOPURINOL-100
Dosage Form : TABLET
Dosage Strength : 100MG
Packaging : 100/1000
Approval Date :
Application Number : 555681
Regulatory Info : Prescription
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?