
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
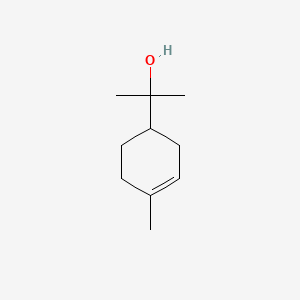

1. 1-alpha-terpineol
2. Alpha-terpineol, Sodium Salt
3. D-alpha-terpineol
4. Dl-alpha-terpineol
5. P-menth-1-en-8-ol
1. Terpineol
2. 98-55-5
3. 2-(4-methylcyclohex-3-en-1-yl)propan-2-ol
4. P-menth-1-en-8-ol
5. 8000-41-7
6. Dl-alpha-terpineol
7. 1-p-menthen-8-ol
8. .alpha.-terpineol
9. Terpineol 350
10. 1-menthene-8-ol
11. Carvomenthenol
12. Terpineols
13. Fema No. 3045
14. 1-methyl-4-isopropyl-1-cyclohexen-8-ol
15. Alpha,alpha,4-trimethyl-3-cyclohexene-1-methanol
16. 2-(4-methyl-3-cyclohexenyl)-2-propanol
17. Terpineol Schlechthin
18. Terpenol
19. Alpha-terpinenol
20. 1-methyl-4-isopropyl-1-cyclohexene-8-ol
21. Mfcd00001557
22. 8006-39-1
23. Chebi:22469
24. 1-alpha-terpineol
25. 21334lvv8w
26. Nsc-21449
27. Nsc-403665
28. 3-cyclohexene-1-methanol, .alpha.,.alpha.,4-trimethyl-
29. Ncgc00164431-01
30. Nsc 21449
31. Pc 593
32. Dsstox_cid_6625
33. Dsstox_rid_79596
34. Dsstox_gsid_40775
35. Terpilenol, Alpha-
36. Terpene Alcohol
37. Fema Number 3045
38. Alpha-terpineol, Analytical Standard
39. Alpha-terpineol (natural)
40. Menth-1-en-8-ol
41. 2-(4-methylcyclohex-3-enyl)propan-2-ol
42. Cas-8000-41-7
43. Ccris 3204
44. 3-cyclohexene-1-methanol,.alpha.4-trimethyl-
45. Caswell No. 823
46. Hsdb 5316
47. Mixture Of P-methenols
48. Einecs 202-680-6
49. Einecs 219-448-5
50. 3-cyclohexene-1-methanol, Alpha,alpha,4-trimethyl-
51. Brn 1906604
52. Unii-r53q4zwc99
53. Alfa_terpineol
54. Unii-21334lvv8w
55. Ai3-00275
56. Terpineol Normal
57. Alpha -terpineol
58. Dl A-terpineol
59. Menthen-8-ol
60. Einecs 232-268-1
61. Epa Pesticide Chemical Code 067005
62. Alfa-terpineol
63. 1-p-menthen-8-
64. Terpineol Or
65. .alpha.terpineol
66. Terpineol, Alpha
67. D-1-p-menthen-8-ol
68. Terpineol, Mixed Isomers
69. Monocyclic Terpenealcohols
70. (+)-.alpha.-terpineol
71. 3-cyclohexene-1-methanol, .alpha.,.alpha.4-trimethyl-
72. Ec 202-680-6
73. Ec 232-268-1
74. Alpha-terpineol, Aldrichcpr
75. Dsstox_rid_78167
76. Dsstox_gsid_26625
77. Pine Oil-593
78. Schembl28466
79. Alpha-terpineol [fcc]
80. 3-cyclohexene-1-methanol, .alpha.,.alpha.,4-trimethyl-, (s)-
81. Alpha-terpineol [hsdb]
82. (1)-alpha,alpha,4-trimethylcyclohex-3-ene-1-methanol
83. Chembl449810
84. R53q4zwc99
85. .alpha.-terpineol [ii]
86. .alpha.-terpineol [mi]
87. Alfa-terpineol [who-dd]
88. Dtxsid5026625
89. Mil-350
90. .alpha.-terpineol [fhfi]
91. Hy-n5142
92. Nsc21449
93. Tox21_112118
94. Tox21_200112
95. Tox21_302298
96. C0669
97. Nsc403665
98. Pc-593
99. 3-cyclohexene-1-methanol, .alpha.,.alpha.,4-trimethyl-, Sodium Salt, (1s)-
100. Akos015840815
101. Alpha-terpineol, 90%, Technical Grade
102. Sb45068
103. Cas-98-55-5
104. Ncgc00248528-01
105. Ncgc00255464-01
106. Ncgc00257666-01
107. Db-059206
108. Alpha-terpineol 1000 Microg/ml In N-hexane
109. Cs-0032554
110. Ft-0622202
111. Ft-0627680
112. Ft-0698995
113. Ft-0772029
114. T0022
115. T0984
116. 2-(4-methyl-1-cyclohex-3-enyl)-propan-2-ol
117. D70165
118. (1r)-a,a,4-trimethyl-3-cyclohexene-1-methanol
119. Sr-01000944873
120. J-500272
121. Sr-01000944873-1
122. Terpin Monohydrate Impurity A [ep Impurity]
123. W-100076
124. Q27109437
125. F0001-2319
126. Alpha-terpineol, Primary Pharmaceutical Reference Standard
127. 3-cyclohexene-1-methanol, Alpha., .alpha., 4-trimethyl-
128. 22347-88-2
| Molecular Weight | 154.25 g/mol |
|---|---|
| Molecular Formula | C10H18O |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 154.135765193 g/mol |
| Monoisotopic Mass | 154.135765193 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 168 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... After iv injection of 0.1 mL/kg, death due to massive pulmonary edema occurred within minutes. In this animal blood and tissue levels of alpha-terpineol of between 150 and 300 ppm were observed. After smaller doses of pine oil (0.033 mL/kg), horses survived until euthanized up to 48 hr later. Blood levels of alpha-terpineol became undetectable in one of these animals after 2 hr, and no tissue levels were detected at postmortem....
PMID:981787 Tobin T et al; Res Commun Chem Pathol Pharm 15 (2): 291 (1976)
The metabolic fate of alpha-terpineol administered orally to male albino-rats was investigated, and its effects on the liver microsomal cytochrome-P-450 system were studied. For metabolic studies, alpha-terpineol was given once daily for 20 days at a dose of 600mg/kg bw; cytochrome-P-450 studies involved dosing for up to 9 days. ...The neutral fraction isolated showed the presence of one major (alpha-terpineol) and two minor compounds. One of the minor compounds was identified as p-menthane-1,2,8-triol. Further study revealed the presence of the methyl esters of oleuropeic-acid and dihydrooleuropeic-acid. Allylic oxidation of C-1 methyl esters appeared to be the major metabolic pathway. It was considered likely that the allylic methyl group at C-7 was oxidized prior to the reduction of the 1,2-double bond. Administration of alpha-terpineol increased the levels of liver microsomal cytochrome-P-450 by 72, 104, 90, 54, and 52% after 1, 2, 3, 6, and 9 days of dosing, respectively. A moderate incr was noted in the levels of liver microsomal NADPH-cytochrome-c-reductase during the first 3 days of repeated dosing. No significant effect was noted on cytochrome-b5 and NADH-cytochrome-c-reductase. The authors conclude that the allylic methyl oxidation of alpha-terpineol is the major route for its metabolic transformation in the rat. The reduction of the endocyclic double bond was specifically noted in the formation of dihydrooleuropeic-acid from oleuropeic-acid.
PMID:3179523 Madyastha KM, Srivatsan V; Bulletin of Environmental Contamination and Toxicology 41 (1): 17-25 (1988)
Biotransformation of alpha-terpineol by the common cutworm (Spodoptera litura) larvae was investigated. alpha-Terpineol was mixed in an artificial diet, and the diet was fed to the larvae (fourth-fifth instar) of S. litura. Metabolites were isolated from the frass and analyzed spectroscopically. Main metabolites were 7-hydroxy-alpha-terpineol (p-menth-1-ene-7,8-diol) and oleuropeic acid (8-hydroxy-p-menth-1-en-7-oic acid). Intestinal bacteria from the frass of larvae did not participate in the metabolism of alpha-terpineol. alpha-Terpineol was preferentially oxidized at the C-7 position (allylic methyl group) by S. litura larvae.
PMID:12166982 Miyazawa M, Ohsawa M; J Agric Food Chem 50 (17): 4916-8 (2002)
Details of the metabolism of alpha-terpineol by Pseudomonas incognita are presented. Degradation of alpha-terpineol by this organism resulted in the formation of a number of acidic and neutral metabolites. Among the acidic metabolites, beta-isopropyl pimelic acid, 1-hydroxy-4-isopropenyl-cyclohexane-1-carboxylic acid, 8-hydroxycumic acid, oleuropeic acid, cumic acid, and p-isopropenyl benzoic acid have been identified. Neutral metabolites identified were limonene, p-cymene-8-ol, 2-hydroxycineole, and uroterpenol. ... /I/t appears that P. incognita degrades alpha-terpineol by at least three different routes. While one of the pathways seems to operate via oleuropeic acid, a second may be initiated through the aromatization of alpha-terpineol. The third pathway may involve the formation of limonene from alpha-terpineol and its further metabolism.
PMID:6525582 Madyastha KM, Renganathan V; Can J Microbiol 30 (12): 1429-36 (1984)
In a minor pathway, the endocyclic alkene of alpha-terpineol is epoxidized and then hydrolysed to yield a triol metabolite 1,2,8-trihydroxy- para-menthane, which was also reported in humans after inadvertent oral ingestion of a pine-oil disinfectant containing alpha-terpineol.
FAO/WHO Joint Expert Committee on Food Additives; WHO Food Additives Series 42: 943. Aliphatic Acyclic and Alicyclic Terpenoid Tertiary Alcohols and Structurally Related Substances (1999). Available from, as of July 14, 2015: https://inchem.org/pages/jecfa.html
Metabolized primarily by conjugation with glucuronic acid and excreted in urine. Oxidation of the allylic methyl group followed by hydrogenation to yield the corresponding saturated acid may occur.
FAO/WHO Joint Expert Committee on Food Additives; WHO Food Additives Series 42: 943. Aliphatic Acyclic and Alicyclic Terpenoid Tertiary Alcohols and Structurally Related Substances (1999). Available from, as of July 14, 2015: https://www.inchem.org/pages/jecfa.html

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
98
PharmaCompass offers a list of Alpha-Terpineol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Alpha-Terpineol manufacturer or Alpha-Terpineol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Alpha-Terpineol manufacturer or Alpha-Terpineol supplier.
PharmaCompass also assists you with knowing the Alpha-Terpineol API Price utilized in the formulation of products. Alpha-Terpineol API Price is not always fixed or binding as the Alpha-Terpineol Price is obtained through a variety of data sources. The Alpha-Terpineol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Alpha-Terpineol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Alpha-Terpineol, including repackagers and relabelers. The FDA regulates Alpha-Terpineol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Alpha-Terpineol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Alpha-Terpineol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Alpha-Terpineol supplier is an individual or a company that provides Alpha-Terpineol active pharmaceutical ingredient (API) or Alpha-Terpineol finished formulations upon request. The Alpha-Terpineol suppliers may include Alpha-Terpineol API manufacturers, exporters, distributors and traders.
click here to find a list of Alpha-Terpineol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Alpha-Terpineol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Alpha-Terpineol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Alpha-Terpineol GMP manufacturer or Alpha-Terpineol GMP API supplier for your needs.
A Alpha-Terpineol CoA (Certificate of Analysis) is a formal document that attests to Alpha-Terpineol's compliance with Alpha-Terpineol specifications and serves as a tool for batch-level quality control.
Alpha-Terpineol CoA mostly includes findings from lab analyses of a specific batch. For each Alpha-Terpineol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Alpha-Terpineol may be tested according to a variety of international standards, such as European Pharmacopoeia (Alpha-Terpineol EP), Alpha-Terpineol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Alpha-Terpineol USP).

