
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Alumina
2. Alumina Ceramic
3. Aluminum Oxide (al130o40)
4. Aluminum Oxide (al2o)
5. Aluminum Oxide (alo2)
6. Bauxite
7. Ceramic, Alumina
8. Corundum
9. Oxide, Aluminum
10. Sapphire
1. Aluminium Oxide
2. 1344-28-1
3. Aluminum Lake
4. Dialuminum;oxygen(2-)
5. Beta-aluminium Oxide
6. Gamma-aluminium Oxide
7. Lmi26o6933
8. 12522-88-2
9. 12737-16-5
10. Fasertonerde
11. Abramant
12. Abramax
13. Abrarex
14. Abrasit
15. Aloxite
16. Alundum
17. Compalox
18. Conopal
19. Faserton
20. Lucalox
21. Martoxin
22. Poraminar
23. Almite
24. Delta Alumina
25. Diadur
26. Saffie
27. Dural
28. Dispal Alumina
29. Theta Alumina
30. Alumina Ceramic
31. Eta-alumina
32. Aluminum Oxide, Mesoporous
33. Aluminum Trioxide
34. Catapal S
35. Jubenon R
36. Microgrit Wca
37. Neobead C
38. Dispal M
39. Hypalox Ii
40. Ketjen B
41. Alumite (oxide)
42. Cab-o-grip
43. Fiber Fp
44. Ludox Cl
45. Aluminite 37
46. Dialuminum Trioxide
47. Alon C
48. Aluminum Sesquioxide
49. Catapal Sb Alumina
50. Alundum 600
51. Dotment 324
52. Dotment 358
53. Gk (oxide)
54. Alcoa F 1
55. Exolon Xw 60
56. A 1 (sorbent)
57. Ps 1 (alumina)
58. Activated Aluminum Oxide
59. F 360 (alumina)
60. G 0 (oxide)
61. G 2 (oxide)
62. Brockmann, Aluminum Oxide
63. Q-loid A 30
64. Aluminum Oxide (brockmann)
65. Khp 2
66. Rc 172dbm
67. Aluminum Oxide (fibrous Forms)
68. Ccris 6605
69. Hsdb 506
70. La 6
71. Aluminum Oxide [nf]
72. Aluminum Oxide (brockmann) (form)
73. Aluminum Oxide (ignited)
74. Einecs 215-691-6
75. Ka 101
76. A1-1401 P(ms)
77. Aluminiumoxid
78. Alpha Alumina
79. Gamma Alumina
80. Unii-lmi26o6933
81. A1-0109 P
82. A1-3916 P
83. A1-3970 P
84. Ai3-02904
85. Double-pass Aao Template 2.5 Cm(d: 100nm,hole Depth: 60 Mum)
86. Activated Alumina
87. Aluminum Oxide G
88. A1-3438 T 1/8''
89. Einecs 254-434-2
90. Nano Aluminum Oxide
91. Aluminum Oxide Gamma
92. Aluminum Oxide, Ar
93. A1-0104 T 3/16''
94. A1-1404 T 3/16''
95. A1-3945 E 1/16''
96. A1-3980 T 5/32''
97. A1-4028 T 3/16''
98. A1-4126 E 1/16''
99. Alumina Nanoparticles
100. Iron Oxide Dispersion
101. Aluminum (ii) Oxide
102. Nano Alumina Dispersion
103. Alumina [hpus]
104. Alumina [inci]
105. Alumina Slurry Polishing
106. Aluminum Oxide Nanowires
107. Aluminium Oxide Nanowires
108. Alumina Sputtering Target
109. Aluminum Oxide Dispersion
110. Aluminium Oxide Dispersion
111. Aluminium Oxide Nanopowder
112. Ec 215-691-6
113. Alumina (alpha) Nanopowder
114. Alumina (gamma) Nanopowder
115. Aluminum Oxide [ii]
116. Aluminum Oxide [mi]
117. Aluminum Oxide [hsdb]
118. Gamma-alumina Oxide Nanopowder
119. Alpha- Alumina Oxide Nanopowder
120. Aluminium Oxide [mart.]
121. Dtxsid1052791
122. Aluminium Oxide [who-dd]
123. Aluminium Oxide Sputtering Target
124. Aluminum Oxide, Sp,99.999%
125. Aluminum Oxide Nanoparticle Disperson
126. Akos030228258
127. Db11342
128. Aluminum Oxide Nanoparticles / Nanopowder
129. Aluminum Oxide Nanopowder / Nanoparticles
130. Large Aperture Aao Template 180-250nm
131. Large Aperture Aao Template 250-300nm
132. Large Aperture Aao Template 300-350nm
133. V Type Aao Template(hole Depth:260 Nm)
134. Aluminum Oxide Nanoparticles, Silane Coated
135. V Type Aao Template(hole Depth :500 Nm)
136. V Type Aao Template(pore Diameter: 450 Nm)
137. Alumina Al2o3 Powder / Aluminum Oxide Powder
138. Aluminum Oxide, Anhydrous [ep Impurity]
139. Double-pass Aao Template 1.2 Cm(hd:400nm)
140. V Type Aao Template(pore Diameter: 90-40 Nm)
141. Zinc Magnesium (zn-mg) Alloy Sputtering Targets
142. Double-pass Aao Template(hd:20-40nm D:1.2cm)
143. Double-pass Aao Template(hd:20-40nm D:2.5cm)
144. Double-pass Aao Template(hd:60-80nm D:1.2cm)
145. Double-pass Aao Template(hd:60-80nm D:2.5cm)
146. Q177342
147. Single-pass Aao Template(hd: 200nm,size:2x2cm)
148. Single-pass Aao Template(hd: 300nm,size:2x2cm)
149. Single-pass Aao Template(hd: 400nm,size:2x2cm)
150. Single-pass Aao Template(hd: 500nm,size:2x2cm)
151. Single-pass Aao Template(hd: 600nm,size:2x2cm)
152. Single-pass Aao Template(hd: 700nm,size:2x2cm)
153. Aluminum Oxide, 99.99% Metals Basis, 56mum, Powder
154. Single-pass Aao Template(thickness:5mum, Hd:300nm)
155. Single-pass Aao Template(thickness: 1mum,hd:5-10 Nm)
156. Single-pass Aao Template(thickness: 50nm,hd:5-10 Nm)
157. Single-pass Aao Template(thickness:60mum, Hd:300nm)
158. Single-pass Aao Template(thickness: 300nm, Hd:40-50 Nm)
159. Single-pass Aao Template(thickness: 300nm, Hd:60-80 Nm)
160. Single-pass Aao Template(thickness: 300nm,hd:20-30 Nm)
161. Single-pass Aao Template(thickness: 5mum, Hd: 60-80 Nm)
162. Single-pass Aao Template(thickness: 5mum,hd:20-30 Nm)
163. Single-pass Aao Template(thickness: 60mum, Hd:20-30 Nm)
164. Single-pass Aao Template(thickness:300nm, Hd:80-100nm)
165. Aluminum Oxide, Activated, Neutral, Brockmann I 58 A Pore Size
166. Single-pass Aao Template Thickness 5mum(d: 50nm, Square 2cm)
167. Single-pass Aao Template(thickness: 60mum, Hd: 60-80 Nm)
168. Al2o3 Alumina Spherical Powder / Aluminum Oxide Spherical Powder
169. Double-pass Aao Template 1.2 Cm(d: 100nm,hole Depth: 50 Mum)
170. Double-pass Aao Template 1.2 Cm(d: 200nm,hole Depth: 50 Mum)
171. Double-pass Aao Template 1.2 Cm(d: 300nm,hole Depth: 50 Mum)
172. Double-pass Aao Template 1.2 Cm(d: 50nm,hole Depth: 50 Mum)
173. Double-pass Aao Template 2.5 Cm(d: 200nm,hole Depth: 60 Mum)
174. Double-pass Aao Template 2.5 Cm(d: 300nm,hole Depth: 60 Mum)
175. Double-pass Aao Template 2.5 Cm(d: 400nm,hole Depth: 60 Mum)
176. Double-pass Aao Template 2.5 Cm(d: 50nm,hole Depth: 60 Mum)
177. Single-pass Aao Template Thickness 50mum(d: 100nm, Square 2cm)
178. Single-pass Aao Template Thickness 50mum(d: 200nm, Square 2cm)
179. Single-pass Aao Template Thickness 50mum(d: 400nm, Square 2cm)
180. Single-pass Aao Template Thickness 50mum(d: 50nm, Square 2cm)
181. Single-pass Aao Template Thickness 5mum(d: 100nm, Square 2cm)
182. Single-pass Aao Template Thickness 5mum(d: 200nm, Square 2cm)
183. Single-pass Aao Template Thickness 5mum(d: 400nm, Square 2cm)
184. Transferable Aao Ultrathin Films 60-70 Nm(hd: 30nm, S:>/=1cm2)
185. Transferable Aao Ultrathin Films 60-70 Nm(hd: 40nm, S:>/=1cm2)
186. Transferable Aao Ultrathin Films 60-70 Nm(hd: 50nm, S:>/=1cm2)
187. Aao Double-pass Filter Membrane 200 Nm(d: 25 Mm, Hole Depth: 200 Nm)
188. Aao Double-pass Filter Membrane 200 Nm(d: 47 Mm, Hole Depth: 200 Nm)
189. Aao Double-pass Filter Membrane 300 Nm(d: 25 Mm, Hole Depth: 300 Nm)
190. Aao Double-pass Filter Membrane 300 Nm(d: 47 Mm, Hole Depth: 300 Nm)
191. Aao Double-pass Filter Membrane 400 Nm(d: 25 Mm, Hole Depth: 400 Nm)
192. Aao Double-pass Filter Membrane 400 Nm(d: 47 Mm, Hole Depth: 400 Nm)
193. Double-pass Aao Template 1.2 Cm(d:40-60nm,hole Depth: 40-70 Mum)
194. Transferable Aao Ultrathin Films 100-110 Nm(hd: 60nm, S:>/=1cm2)
195. Transferable Aao Ultrathin Films 100-110 Nm(hd: 70nm, S:>/=1cm2)
196. Transferable Aao Ultrathin Films 100-110 Nm(hd: 80nm, S:>/=1cm2)
197. Transferable Aao Ultrathin Films 100-110 Nm(hd: 90nm, S:>/=1cm2)
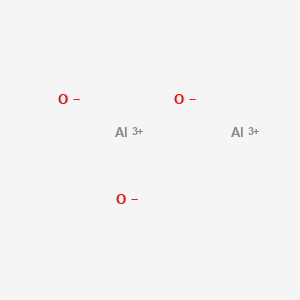
| Molecular Weight | 101.961 g/mol |
|---|---|
| Molecular Formula | Al2O3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 101.947821 g/mol |
| Monoisotopic Mass | 101.947821 g/mol |
| Topological Polar Surface Area | 3 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AX - Other anti-acne preparations for topical use
D10AX04 - Aluminium oxide
Inhalation exposure to 100 mg/hr aluminium, in the form of powder, or 92 mg Al/ per 2 hr, as a fume, each day for 9-13 months showed a significant retention of aluminium in the lungs of both groups of animals. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Following exposure to fresh air, aluminium oxide was cleared rapidly from the lungs of the both powder and fume groups. Weight of wet lung, ash and aluminium oxide content of lungs in exposed animals increased. The initial pulmonary tissue response was proliferation of macrophages within alveolar spaces as well as lipoid pneumonia. The focal aggregates of macrophages were located around the small bronchioles and small pulmonary arterioles; lymphoid hyperplasia was observed. After chronic exposure to aluminium powder, rats showed focal deposits of hyaline in alveolar walls, and focal areas of lipoid pneumonia developed in hamsters.
Krewski D et al; J Toxicol Environ health B Crit Rev 10 (Suppl 1): 1-269 (2007)
... The concentrations of aluminum in tissues of female New Zealand rabbits exposed to /aluminum oxide/ dust at a concentration of 0.56 mg Al/cu m for 5 months (8 hr/day, 5 days/week) were determined. The amount of aluminum in the brains of the animals was nearly two and a half times as high as that of the control animals. The concentrations in other tissues were only slightly increased.
European Commission, ESIS; IUCLID Dataset, Aluminum oxide (1344-28-1) p.66 (2000 CD-ROM edition). Available from, as of April 26, 2010: https://esis.jrc.ec.europa.eu/
Radiolabeled 26Al inhalation studies were performed using tagged gamma aluminum oxide. The results showed that the material acted as an insoluble dust. about 45% was cleared from the lung after one day, with slow mechanical clearance from the lungs of the balance. After 72 days, only 0.2% of the alumina remained in the lung. The lung may provide long-term sequestration for small percentage of inhaled, insoluble alumina.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-1634 2007.
/Investigators/ evaluated the accumulation and clearance of aluminum oxide in Sprague-Dawley rats following intratracheal instillation. Rats were instilled with 1 mg/kg of smelter grade alumina obtained via electrostatic precipitator (MMAD = 1.2 um) once per week for 20 weeks. Groups of rats were sacrificed periodically during the exposure period and up to 19-weeks post exposure. Aluminum was measured by flame and flameless atomic absorption spectroscopy. Exposed rats steadily accumulated aluminum during the exposure period up to a lung burden of about 500 ug Al/g tissue.Only 9% was cleared during the 19-week post-exposure period. Extrapulmonary tissues such as brain, bone, kidney, liver, and spleen had aluminum levels that were essentially the same as, but slightly above, those of non-exposed control animals (0.17-2.63 ug Al/g tissue.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-1634 2007.
For more Absorption, Distribution and Excretion (Complete) data for Aluminum oxide (6 total), please visit the HSDB record page.
The mean plasma half-life of aluminum after iv admin in dogs is approx 4.5 hr. /Aluminum/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 1009
The shorter half-life for the urinary elimination of aluminum was about 8 hr. /Aluminum/
Friberg, L., Nordberg, G.F., Kessler, E. and Vouk, V.B. (eds). Handbook of the Toxicology of Metals. 2nd ed. Vols I, II.: Amsterdam: Elsevier Science Publishers B.V., 1986., p. 10
... Six 8 wk old female Swiss Webster mice were fed for 10 weeks purified diets containing 100 (control), 500 or 1000 ug aluminum/g diet. Brain and liver lipid peroxidation was determined by evaluating the production of 2-thiobarbituric acid reactive substances in brain and liver homogenates in the presence or absence of 50 uM ferrous iron. 2-Thiobarbituric acid reactive substances production in the absence of iron in brain homogenates from mice fed the 1000 ... ug/g diet was higher (30%) than that in the ... control group (3.1 vs 2.4 nmol 2-thiobarbituric acid reactive substances/mg protein). The addition of ferrous iron increased 2-thiobarbituric acid reactive substances production in brain homogenates from all 3 dietary groups. The iron induced 2-thiobarbituric acid reactive substances production was 26% higher in the 1000 ... ug/g /group/ brain homogenates than in the ... /control/ group (4.9 vs 3.9 nmol 2-thiobarbituric acid reactive subtances/mg protein). Brain 2-thiobarbituric acid reactive substances production in the presence and absence of iron was similar between the 100 and 500 ... ug/g /diet/ groups. 2-Thiobarbituric acid reactive substances production in liver homogenates measured either with or without iron was similar for the 3 groups. These results show that, in mice, dietary aluminum intoxication leads to increased brain 2-thiobarbituric acid reactive substance production, suggesting that enhanced lipid peroxidation may be one possible mechanism underlying the neurological damage associated with increased tissue aluminum. /Aluminum/
PMID:2330606 Fraga CG et al; Toxicol Lett 51 (2): 213-9 (1990)
Evidence is presented indicating that dementias are associated with a relative insufficiency of magnesium in the brain. Such insufficiency may be attributable to low intake or retention of magnesium; high intake of a neurotoxic metal, such a aluminum, which inhibits activity of magnesium requiring enzymes; or impaired transport of magnesium and/or enhanced transport of the neurotoxic metal into brain tissue. It is proposed that Alzheimer's disease involves a defective transport process, characterized by both an abnormally high incorporation of aluminum and an abnormally low incorporation of magnesium into brain neurons. The hypothesis has advanced that an altered serum protein contributes to the progression of Alzheimer's disease by having a greater affinity for aluminum than for magnesium, in contrast to the normal protein, which binds magnesium better than aluminum. The altered protein crosses the blood-brain barrier more efficiently than the normal protein and competes with the normal protein in binding to brain neurons. Binding of the altered protein to the target neurons would both facilitate aluminum uptake and impede magnesium uptake. Evidence suggests that albumin is the serum protein that is altered. /Aluminum/
PMID:2092675 Glick JL; Med Hypotheses 31 (3): 211-25 (1990)
Aluminum is established as a neurotoxin, although the basis for its toxicity is unknown. It recently has been shown to alter the function of the blood brain barrier, which regulates exchanges between the central nervous system and peripheral circulation. The blood brain barrier owes its unique properties to the integrity of cell membranes that comprise it. Aluminum affects some of the membrane-like functions of the blood brain barrier. It increases the rate of transmembrane diffusion and selectively changes saturable transport systems without disrupting the integrity of the membranes or altering CNS hemodynamics. Such alterations in the access to the brain of nutrients, hormones, toxins, and drugs could be the basis of CNS dysfunction. Aluminum is capable of altering membrane function at the blood-brain barrier; many of its effects on the CNS as well as peripheral tissues can be explained by its actions as a membrane toxin. /Aluminum/
PMID:2671833 Banks WA, Kastin AJ; Neurosci Biobehav Rev 13 (1): 47-5 (1989)
Chronic, oral administration of aluminum to rats increases the in vivo concentration of cyclic AMP and the phosphorylation of microtubule-associated protein-2 and the 200 kd neurofilament subunit. In the present study, the effect of this treatment on endogenous protein phosphorylation in soluble and particulate fractions prepared from cerebral cortices was examined. Chronic aluminum treatment significantly elevated the basal and cyclic AMP-dependent phosphorylation of 11-12 endogenous proteins in the soluble fraction prepared from cerebral cortices. Endogenous protein phosphorylation in the soluble fraction occurring in the presence of calcium(+2) alone or calcium(+2) phorbol 12-myristate 13-acetate and phosphatidylserine was not significantly altered by aluminum treatment. In the particulate fraction the phosphorylation of several proteins was significantly decreased by aluminum administration; however the phosphorylation of the majority of protein substrates remained unaltered. Aluminum treatment did not alter the activities of cyclic AMP-dependent protein kinase or protein tyrosine kinase in the soluble and particulate fractions. The activity of calcium(+2)/phospholipid-dependent protein kinase (protein kinase C) was increased in the particulate fraction of aluminum fed rats. These results clearly demonstrate that specific effects on protein kinase activities result from in vivo aluminum administration. /Aluminum/
Johnson GVW et al; Neuroiol Aging 11 (3): 209-16 (1990)
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
94
PharmaCompass offers a list of Aluminium Oxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aluminium Oxide manufacturer or Aluminium Oxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aluminium Oxide manufacturer or Aluminium Oxide supplier.
PharmaCompass also assists you with knowing the Aluminium Oxide API Price utilized in the formulation of products. Aluminium Oxide API Price is not always fixed or binding as the Aluminium Oxide Price is obtained through a variety of data sources. The Aluminium Oxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aluminium Oxide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aluminium Oxide, including repackagers and relabelers. The FDA regulates Aluminium Oxide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aluminium Oxide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aluminium Oxide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aluminium Oxide supplier is an individual or a company that provides Aluminium Oxide active pharmaceutical ingredient (API) or Aluminium Oxide finished formulations upon request. The Aluminium Oxide suppliers may include Aluminium Oxide API manufacturers, exporters, distributors and traders.
click here to find a list of Aluminium Oxide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aluminium Oxide written confirmation (Aluminium Oxide WC) is an official document issued by a regulatory agency to a Aluminium Oxide manufacturer, verifying that the manufacturing facility of a Aluminium Oxide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Aluminium Oxide APIs or Aluminium Oxide finished pharmaceutical products to another nation, regulatory agencies frequently require a Aluminium Oxide WC (written confirmation) as part of the regulatory process.
click here to find a list of Aluminium Oxide suppliers with Written Confirmation (WC) on PharmaCompass.
Aluminium Oxide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aluminium Oxide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aluminium Oxide GMP manufacturer or Aluminium Oxide GMP API supplier for your needs.
A Aluminium Oxide CoA (Certificate of Analysis) is a formal document that attests to Aluminium Oxide's compliance with Aluminium Oxide specifications and serves as a tool for batch-level quality control.
Aluminium Oxide CoA mostly includes findings from lab analyses of a specific batch. For each Aluminium Oxide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aluminium Oxide may be tested according to a variety of international standards, such as European Pharmacopoeia (Aluminium Oxide EP), Aluminium Oxide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aluminium Oxide USP).
