
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
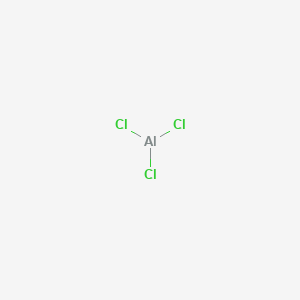

1. Alcl3
2. Aluminum Chloride Hexahydrate
3. Aluminum Chloride, Anhydrous
4. Aluminum Trichloride
5. Anhydrous Aluminum Chloride
6. Drysol
1. Aluminum Trichloride
2. Aluminium Chloride
3. 7446-70-0
4. Aluminium Trichloride
5. Trichloroalumane
6. Trichloroaluminum
7. Alcl3
8. Aluminum Chloride (alcl3)
9. Aluminum Chloride Anhydrous
10. Aluminiumchlorid
11. Aluminum Chloride (1:3)
12. Aluminum Chloride, Anhydrous
13. Chlorure D'aluminium
14. Alluminio(cloruro Di)
15. Aluminium, (chlorure D')
16. Aluminium(ii)chloride
17. Aluminum Chloride, Anhydrous, Powder
18. Mfcd00003422
19. Nsc-143015
20. Nsc-143016
21. Wln: Al G3
22. Pearsall
23. Tk Flock
24. Caswell No. 029
25. Aluminiumchlorid [german]
26. Aluminum Chloride - Ethanol Solution
27. Hemoban
28. Aluminum Chloride (anhydrous)
29. Chlorure D'aluminium [french]
30. Ccris 6871
31. Hsdb 607
32. Alluminio(cloruro Di) [italian]
33. Trichloridoaluminium
34. Aluminum, (chlorure D') [french]
35. Aluminium, (chlorure D') [french]
36. Einecs 231-208-1
37. Un1726
38. Un2581
39. Epa Pesticide Chemical Code 013901
40. Nsc 143015
41. Nsc 143016
42. Aluminium(3+) Chloride
43. Ai3-01917
44. [alcl3]
45. Aluminum, (chlorure D')
46. Aluminum Chloride, Solution
47. Unii-lif1n9568y
48. Aluminum Chloride, Ultra Dry
49. Aluminium Chloride, Anhydrous
50. Chembl3833401
51. Dtxsid6029674
52. Chebi:30114
53. Bcp21020
54. Nsc143015
55. Nsc143016
56. Aluminum Chloride, Puriss., 98.0%
57. Akos015902776
58. Aluminium Chloride, Anhydrous, Reagent
59. Aluminum Chloride, Anhydrous, Granular
60. Aluminum Chloride, Reagent Grade, 98%
61. Db11081
62. Ncgc00180999-01
63. Aluminum Chloride, Reagentplus(r), 99%
64. Bp-10698
65. Aluminum Chloride, 6.0n Standardized Solution
66. Aluminum Chloride, 99.99% Trace Metals Basis
67. Aluminum Chloride, Anhydrous, Sublimed, >=98%
68. Aluminum Chloride, Saj First Grade, >=98.0%
69. Ec 231-208-1
70. Aluminum Chloride Solution, 1.0 M In Nitrobenzene
71. Q314036
72. Aluminum Chloride, Solution [un2581] [corrosive]
73. Aluminum Chloride, Anhydrous [un1726] [corrosive]
74. Aluminum Chloride, Purum, Anhydrous, >=98.0% (at)
75. Aluminum Chloride, 99.98% Trace Metals Grade Anhydrous, Powder
76. Aluminum Chloride, Anhydrous, Powder, 99.99% Trace Metals Basis
77. Aluminum Chloride, Anhydrous, Powder, 99.999% Trace Metals Basis
78. Aluminum Standard For Aas, Analytical Standard, Traceable To Bam
79. Aluminum Chloride, Reagentplus(r), Anhydrous, >=99.9% Trace Metals Basis
80. Aluminum Chloride - Ethanol Solution, ~11% In Ethanol/water, For Tlc Derivatization
81. Aluminum Chloride, Anhydrous, Crystallized 4-6 Mesh Size (crystalline Chunks)
82. Aluminum Atomic Spectroscopy Standard Concentrate 1.00 G Al, 1.00 G/l, For 1 L Standard Solution, Analytical Standard
83. Aluminum Atomic Spectroscopy Standard Concentrate 10.00 G Al, 10.00 G/l, For 1 L Standard Solution, Analytical Standard
| Molecular Weight | 133.34 g/mol |
|---|---|
| Molecular Formula | AlCl3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 131.888096 g/mol |
| Monoisotopic Mass | 131.888096 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Astringents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Some aluminum compounds are employed therapeutically, eg, aluminum hydroxide is one component of the antacids recommended in the treatment of stomach ulcers and gastritis. Large doses of aluminum hydroxide (in the order of grams) are prescribed for patients who, as a result of renal dysfunction, have high blood phosphate levels. Aluminum acetotartrate in solution is used in the treatment of sores and for other dermatological purposes. The solution inhibits bacteria and has astringent properties. Aluminum chloride hexahydrate is very commonly used in deodorants, and a solution of aluminum sulfate has been tried without significant success against stings of fire ants. /Aluminum chloride hexahydrate/
Friberg, L., Nordberg, G.F., Kessler, E. and Vouk, V.B. (eds). Handbook of the Toxicology of Metals. 2nd ed. Vols I, II.: Amsterdam: Elsevier Science Publishers B.V., 1986., p. 4
Antiperspirant
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 949
Anhydrotic. /Aluminum chloride hexahydrate/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 61
Aluminum chloride shown to exhibit good antiperspirant action in pilocarpine-induced sweating in rat foot pads and this result is in accordance with observations in human tests.
LANSDOWN ABG; J SOC COSMET CHEM 24 (OCT 14th): 677-684 (1972)
- Indicated for the control of minor hemorrhage during dental restorative procedures. - Indicated to reduce underarm perspiration.
Aluminum chloride is a hemostatic and antiperspirant agent.
Antiperspirants
Agents that are put on the SKIN to reduce SWEATING or prevent excess sweating (HYPERHIDROSIS). (See all compounds classified as Antiperspirants.)
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AX - Other anti-acne preparations for topical use
D10AX01 - Aluminium chloride
Absorption
It is reported that about 17-30 % of aluminum chloride formed from the reaction between orally ingested aluminum hydroxide and hydrochloric acid of the stomach is absorbed. In rabbits, administration of a single maximum safe oral dose aluminum chloride (333 mg Al/kg) resulted in aluminum absorption of 0.57 %. Aluminum chloride may be absorbed via dermal route, with the uptake increasing in the microgram range but with an upper limit.
Route of Elimination
Absorbed aluminum chloride is rapidly excreted by the kidneys in individuals with normal renal function. Aluminum chloride may be absorbed dermally.
Volume of Distribution
Following oral administration of 8.1 mg/kg of aluminum chloride, the steadystate volume of distribution of aluminum was 38.4 6.4 mL/kg.
Clearance
Following oral administration of 8.1 mg/kg of aluminum chloride, the clearance of aluminum was 8.87 1.76 mL/(h*kg).
Rainbow trout (9 to 220 g) were individually placed in 1 liter chambers having aerated, fresh flowing water for 1 hr (control conditions, aluminum concentration= 0.033 mg/L, pH 4.61). They were then exposed to an aerated artificial medium (flow rate= 297 + or - 11 mL/min, pH= 5.4) containing 0.954 + or - 0.133 mg/L aluminum (as aluminum chloride) for up to 1 hr. There was no significant difference (p= 0.05) between episodic aluminum levels and episodic blank (no fish) aluminum levels, indicating that the flow rate through the chambers was sufficient to maintain aluminum levels such that absorption of aluminum from the apparatus was negligible. Fish were removed for tissue sampling after 5, 10, 20, 30, and 60 min exposure. Skin and blood showed no significant increase in aluminum content (p=0.05). Gill tissue episodic aluminum values were significantly higher (p=0.05)) than control levels at 30 and 60 min, having increased from 8 ug/g to 50 ug/g wet weight in 1 hr. Mucous aluminum content was significantly higher (p=0.05) than control values after 5 min of exposure increased to 4.5 mg/L from 0.2 mg/L. Mucous cells on the secondary lamellae showed discharged mucous globules on the gill surface after 1 hr of episodic exposure, a feature not shown by unexposed gill lamellae.
Handy RD, Eddy FB; J Fish Biol 34 (6): 865-74 (1989)
Aluminum hydroxide or oxide is slowly solubilized in the stomach and reacts with hydrochloric acid to form aluminum chloride and water. In addition to forming aluminum chloride, dihydroxyaluminum sodium carbonate and aluminum carbonate form carbon dioxide, and aluminum phosphate forms phosphoric acid. About 17-30% of the aluminum chloride formed is absorbed and is rapidly excreted by the kidneys in patients with normal renal functions. In the small intestine, aluminum chloride is rapidly converted to insoluble poorly absorbed basic aluminum salts which probably /include/ a mixture of hydrated aluminum oxide, oxyaluminum hydroxide, various basic aluminum carbonates, and aluminum soaps. Aluminum-containing antacids (except aluminum phosphate) also combine with dietary phosphate in the intestine forming insoluble, nonabsorbable aluminum phosphate which is excreted in the feces. If phosphate intake is limited in patients with normal renal function, aluminum antacids (except aluminum phosphate) decrease phosphate absorption and hypophosphatemia and hypophosphaturia occur; calcium absorption is increased. In vitro studies indicate that aluminum hydroxide binds bile salts with an affinity & capacity similar to that of cholestyramine; aluminum phosphate binds bile salts, but to a much lesser degree than does aluminum hydroxide. /Aluminum antacids/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2758
Evidence of transdermal uptake of aluminum was reported... when /aluminum chloride/ was chronically placed on the skin of mice. Uptake increased in the microgram range and appeared to have an upper limit, failing to increase at larger doses. ...A similar finding was reported when /aluminum chloride/ was placed in the nasal passages of experimental animals; apparently aluminum wa translocated to the olfactory region of the brain. However, the chemically irritant nature of this mode of administration and the potential damage to the mucosa so treated was not factored into the evaluation of the integrity of this barrier.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V2 370
Following a single maximum safe oral dose of the water soluble compounds aluminum chloride (333 mg Al/kg), aluminum nitrate (934 mg Al/kg), aluminum citrate (1,081 mg Al/kg), and aluminum lactate (2,942 mg Al/kg) in rabbits, aluminum absorption was 0.57, 1.16, 2.18, and 0.63%, respectively.
DHHS/ATSDR; Toxicological Profile for Aluminum (July 1999). Available from, as of May 21, 2004: https://www.atsdr.cdc.gov/toxprofiles/index.asp
For more Absorption, Distribution and Excretion (Complete) data for ALUMINUM CHLORIDE (12 total), please visit the HSDB record page.
In the small intestine, aluminum chloride is rapidly converted to insoluble poorly absorbed basic aluminum salts, consisting of a mixture of hydrated aluminum oxide, oxyaluminum hydroxide, various basic aluminum carbonates, and aluminum soaps.
Following oral administration of 8.1 mg/kg of aluminum chloride, the half-life of aluminum was 5.29 0.47 h.
Aluminum chloride is commonly used topical antiperspirant. It is proposed that aluminum chloride works by causing an obstruction of the distal sweat gland ducts, where the metal ions precipitate with mucopolysaccharides, damaging epithelial cells along the lumen of the duct and forming a plug that blocks sweat output. Aluminum chloride is also an astringent that promotes hemostasis; it precipitates proteins on the superficial layer of mucosa and make it mechanically stronger. It creates superficial and local coagulation in minor hemorrhages.
Aluminum (10, 12.5, 17.5, 25 and 50 uM, as aluminum trichloride) inhibited yeast glucose-6-phosphate dehydrogenase (EC 1.1.1.49) by a pseudo-first-order reaction. The inhibition was proportional to the incubation time (10 to 60 sec) and the concn of aluminum. Aluminum was a better inhibitor when added to the buffered enzyme prior to the addition of glucose-6-phosphate or NADP+. When aluminum trichloride was added to the buffered mixture of enzyme and glucose-6-phosphate , more than 10 times aluminum trichloride was needed to observe a comparable inhibition. The inhibitory effect of aluminum chloride on glucose-6-phosphate dehydrogenase was negligible when aluminum chloride was premixed with NADP+. Double reciprocal plots gave a straight line with a k(inact) of 8.3/min and indicated the presence of a binding step prior to inhibition. The kinetic study showed that 1 mol of aluminum was bound per mol of enzyme subunit. A marked incr in sensitivity to aluminum was observed as the pH decr. An inhibitory effect of aluminum was predominant below pH 7.0, but above pH 8.0, aluminum did not significantly affect the reaction rate of glucose-6-phosphate dehydrogenase.
PMID:2665187 Cho SW, Joshi JG; Toxicol Lett 47 (3): 215-9 (1989)
The effect of aluminum on intestinal calcium absorption was determined in male Sprague-Dawley rats using an everted intestinal sac technique. Bidirectional calcium flux in the duodena and ilea of normal rats was assessed by means of dual calcium isotopes. Addition of 2 uM aluminum (as aluminum chloride) to the buffer solution significantly inhibited net calcium absorption in the duodenum through suppression of mucosa-to-serosa flux. Serosa-to-mucosa calcium flux was not similarly influenced by aluminum. In the ileum, aluminum had no effect on any component of calcium flux. Aluminum did not induce any suppression of glucose transport in either the duodenum or ileum, suggesting that the effect on calcium transport is relatively specific.
Adler AJ et al; Am J Physiol 257 (3,1): G433-7 (1989)
ATP pools extracted from the cyanobacterium Anabaena cylindrica, grown in the absence or presence of aluminum chloride were measured using the luciferin-luciferase assay. Addition of low concn of aluminum chloride (3.6-36 uM) incr the ATP pool 20-40% within 24 hr, the effect being more marked with time. When using the Tris-EDTA boiling technique for extraction of cellular ATP, the ATP from aluminum-exposed cells appeared more stable during the extraction than the ATP from untreated cells. The higher ATP pools in aluminum-exposed cells were also evident after dark treatment and addition of the phosphorylating inhibitors carbonylcyanide m-chlorophenylhydrazone and N,N'-dicyclohexylcarbodiimide. The formation of elevated ATP pools in cells exposed to aluminum was curtailed by high concn of cellular phosphate and postincubation at high pH (> 8).
Pettersson A, Bergman B; Physiol Plant 76 (4): 527-34 (1989)
Very few investigations have been made on the metabolism and mode of action of aluminum compounds for the reason that it is very poorly absorbed and of low toxicity. The existing evidence indicates that the small portion of aluminum ion that hydrolyzes combines with available phosphate, becomes insoluble and unabsorbed, and so is excreted along with the un-ionized portion. In high doses aluminum compounds have been shown to affect phosphorus metabolism of rats and mice. At lower levels (170 and 355 ppm aluminum as aluminum chloride) aluminum balance studies showed intake and fecal excretion of aluminum were higher at the higher dose, but urinary excretion and retention were not. In phosphorus balance studies made at 160 to 180 and 355 ppm in the diet, the higher dose lowered phosphorus retention, although phosphorus content of the liver and femur were not affected. Chronic and acute poisoning by aluminum chloride caused, on intraperitoneal administration of (32)H-labelled disodium hydrogen phosphate, decreased incorporation of (32)phosphorus in the phospholipids and nucleic acids of various rat tissues. Decreased adenosine triphosphate levels and a rise in the adenosine diphosphate level in plasma also occurred, indicating interference with tissue phosphorylation process.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 1500
For more Mechanism of Action (Complete) data for ALUMINUM CHLORIDE (10 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
62
PharmaCompass offers a list of Aluminum Chloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aluminum Chloride manufacturer or Aluminum Chloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aluminum Chloride manufacturer or Aluminum Chloride supplier.
PharmaCompass also assists you with knowing the Aluminum Chloride API Price utilized in the formulation of products. Aluminum Chloride API Price is not always fixed or binding as the Aluminum Chloride Price is obtained through a variety of data sources. The Aluminum Chloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aluminum Chloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aluminum Chloride, including repackagers and relabelers. The FDA regulates Aluminum Chloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aluminum Chloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aluminum Chloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aluminum Chloride supplier is an individual or a company that provides Aluminum Chloride active pharmaceutical ingredient (API) or Aluminum Chloride finished formulations upon request. The Aluminum Chloride suppliers may include Aluminum Chloride API manufacturers, exporters, distributors and traders.
click here to find a list of Aluminum Chloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aluminum Chloride written confirmation (Aluminum Chloride WC) is an official document issued by a regulatory agency to a Aluminum Chloride manufacturer, verifying that the manufacturing facility of a Aluminum Chloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Aluminum Chloride APIs or Aluminum Chloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Aluminum Chloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Aluminum Chloride suppliers with Written Confirmation (WC) on PharmaCompass.
Aluminum Chloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aluminum Chloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aluminum Chloride GMP manufacturer or Aluminum Chloride GMP API supplier for your needs.
A Aluminum Chloride CoA (Certificate of Analysis) is a formal document that attests to Aluminum Chloride's compliance with Aluminum Chloride specifications and serves as a tool for batch-level quality control.
Aluminum Chloride CoA mostly includes findings from lab analyses of a specific batch. For each Aluminum Chloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aluminum Chloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Aluminum Chloride EP), Aluminum Chloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aluminum Chloride USP).
