
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
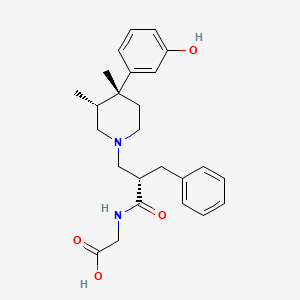

1. Adl 8-2698
2. Adl8-2698
3. Alvimopan Anhydrous
4. Anhydrous Alvimopan
5. Entereg
6. Ly 246736
7. Ly-246736
8. Ly246736
9. Trans-3,4-dimethyl-4-(3-hydroxyphenyl) Piperidine
1. 156053-89-3
2. Alvimopan Anhydrous
3. Adl 8-2698
4. Anhydrous Alvimopan
5. Alvimopan [inn]
6. Ly 246736
7. Ly246736
8. 145590-44-9
9. 2-((s)-2-benzyl-3-((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propanamido)acetic Acid
10. 2-[[(2s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl]amino]acetic Acid
11. Q153v49p3z
12. Ly-246736
13. Glycine, N-[(2s)-2-[[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-1-oxo-3-phenylpropyl]-
14. ((s)-2-benzyl-3-((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propanoyl)glycine
15. N-[(2s)-2-{[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-3-phenylpropanoyl]glycine
16. Unii-q153v49p3z
17. Trans-3,4-dimethyl-4-(3-hydroxyphenyl) Piperidine
18. Glycine, N-((2s)-2-(((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-
19. Alvimopan [mi]
20. Alvimopan [mart.]
21. Alvimopan [who-dd]
22. Schembl49578
23. Chembl270190
24. Gtpl7471
25. Alvimopan, >=98% (hplc)
26. Hsdb 7704
27. Dtxsid60166035
28. Chebi:135686
29. Hms3886b09
30. Anhydrous Alvimopan [mart.]
31. Zinc3802417
32. Bdbm50088381
33. Mfcd09838268
34. S5935
35. Akos015896476
36. Cs-0536
37. Db06274
38. Ncgc00378640-01
39. Ncgc00378640-02
40. Ncgc00378640-03
41. As-35088
42. Hy-13243
43. 053a893
44. Sr-01000945029
45. Q4738021
46. Sr-01000945029-1
47. (((2s)-2-(((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)methyl)-3-phenylpropanoyl)amino)acetic Acid
48. ((2(s)-((4(r)-(3-hydroxyphenyl)-3(r),4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)amino)acetic Acid
49. [[(s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propionyl]amino]acetic Acid
50. 2-((s)-2-benzyl-3-((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propanamido)aceticacid
51. 2-[(2s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanamido]acetic Acid
52. 2-[[(2s)-2-[[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl]-3-phenylpropanoyl]amino]acetic Acid
53. Glycine, N-(2-((4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-, (3r-((s*),3.alpha.,4.alpha.))-
54. N-((2s)-2-(((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)glycine
55. Ng0
| Molecular Weight | 424.5 g/mol |
|---|---|
| Molecular Formula | C25H32N2O4 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 424.23620751 g/mol |
| Monoisotopic Mass | 424.23620751 g/mol |
| Topological Polar Surface Area | 89.9 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Entereg |
| PubMed Health | Alvimopan (By mouth) |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | ENTEREG Capsules contain alvimopan, aperipherally-acting -opioid receptor (PAM-OR) antagonist. Chemically, alvimopan is the single stereoisomer [[2(S)-[[4(R)-(3-hydroxyphenyl)-3(R),4-dimethyl-1-piperidinyl]methyl]-1-oxo-3-phenylpropyl]amino]aceti... |
| Active Ingredient | Alvimopan |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 12mg |
| Market Status | Prescription |
| Company | Cubist Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Entereg |
| PubMed Health | Alvimopan (By mouth) |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | ENTEREG Capsules contain alvimopan, aperipherally-acting -opioid receptor (PAM-OR) antagonist. Chemically, alvimopan is the single stereoisomer [[2(S)-[[4(R)-(3-hydroxyphenyl)-3(R),4-dimethyl-1-piperidinyl]methyl]-1-oxo-3-phenylpropyl]amino]aceti... |
| Active Ingredient | Alvimopan |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 12mg |
| Market Status | Prescription |
| Company | Cubist Pharms |
Gastrointestinal Agents
National Library of Medicine's Medical Subject Headings. Alvimopan. Online file (MeSH, 2014). Available from, as of January 30, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Entereg is indicated to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection surgery with primary anastomosis. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
Although alvimopan has been studied for the management of postoperative ileus in women undergoing total abdominal hysterectomy under general anesthesia, efficacy of the drug for this indication has not been established to date. /NOT included in US product label/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3036
/EXPL/ Our objective was to investigate the efficacy and safety of alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist, in subjects with non-cancer pain and opioid-induced bowel dysfunction (OBD), and to identify at least one treatment regimen that improves OBD. Following a 2-week baseline period, 522 subjects reporting <3 spontaneous bowel movements (SBMs)/week (with >or= 25% accompanied by a sensation of incomplete evacuation, straining, or lumpy hard stools), requiring analgesia equivalent to >or= 30 mg oral morphine/day were randomized to alvimopan 0.5 mg twice daily (BID), 1mg once daily (QD), 1 mg BID, or placebo for 6 weeks. Compared with placebo, there was a statistically and clinically significant increase in mean weekly SBM frequency over the initial 3 weeks of treatment (primary endpoint) with alvimopan 0.5 mg BID (+1.71 mean SBMs/week), alvimopan 1mg QD (+1.64) and alvimopan 1 mg BID (+2.52); P<0.001 for all comparisons. Increased SBM frequency and additional treatment effects, including improvements in symptoms such as straining, stool consistency, incomplete evacuation, abdominal bloating/discomfort, and decreased appetite, were sustained over 6 weeks. The most frequently reported adverse events were abdominal pain, nausea, and diarrhea, occurring more frequently in the higher dosage groups. The alvimopan 0.5 mg BID regimen demonstrated the best benefit-to-risk profile for managing OBD with alvimopan in this study population, with a side effect profile similar to that of placebo. There was no evidence of opioid analgesia antagonism. Competitive peripheral antagonism of opioids with alvimopan can restore GI function and relieve OBD without compromising analgesia.
PMID:18164818 Webster L et al; Pain 137 (2): 428-40 (2008)
/BOXED WARNING/ WARNING: POTENTIAL RISK OF MYOCARDIAL INFARCTION WITH LONG-TERM USE: FOR SHORT-TERM HOSPITAL USE ONLY. There was a greater incidence of myocardial infarction in alvimopan-treated patients compared to placebo-treated patients in a 12-month clinical trial, although a causal relationship has not been established. In short-term trials with Entereg, no increased risk of myocardial infarction was observed. Because of the potential risk of myocardial infarction with long-term use, Entereg is available only through a restricted program for short-term use (15 doses) under a Risk Evaluation and Mitigation Strategy (REMS) called the Entereg Access Support and Education (E.A.S.E.) Program [see
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (Updated: October 2014). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=77a67dc6-35d3-48ff-9d18-292d4d442f70
A numerically higher incidence of myocardial infarction was reported in patients receiving alvimopan 0.5 mg twice daily compared with placebo in a 12-month clinical study evaluating long-term use of the drug for management of opiate-induced bowel dysfunction in patients with chronic pain; a majority of events occurred 1 - 4 months after initiation of therapy. Similar results have not been observed in patients receiving short-term alvimopan therapy (12 mg twice daily for 7 days or less) following bowel resection. A causal relationship between myocardial infarction and alvimopan has not been established. Because of an increased risk of ischemic cardiac events with long-term therapy, alvimopan is available only to hospitals through a restricted distribution program (EASE program).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3037
Use of alvimopan in patients undergoing surgical correction of complete bowel obstruction is not recommended.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3037
Increased variability in alvimopan pharmacokinetics has been observed in patients with active or quiescent Crohn's disease; exposure to the drug generally was twofold higher in patients with quiescent disease compared with healthy individuals or patients with active disease. Concentrations of alvimopan's metabolite were lower in patients with Crohn's disease. However, the manufacturer states that dosage adjustments are not necessary.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3037
For more Drug Warnings (Complete) data for ALVIMOPAN (12 total), please visit the HSDB record page.
Used to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection surgery with primary anastomosis. Also investigated for use in the treatment of pain (acute or chronic).
FDA Label
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AH - Peripheral opioid receptor antagonists
A06AH02 - Alvimopan
Absorption
Alvimopan's high affinity for the peripheral mu-receptor leads to slower absorption dependent on dissociation from the receptor and subsequently low oral bioavailability of less than 7%.
Route of Elimination
Biliary secretion was considered the primary pathway for alvimopan elimination. Unabsorbed drug and unchanged alvimopan resulting from biliary excretion were then hydrolyzed to its metabolite by gut microflora. Feces (via biliary excretion) & urine (35%)
Volume of Distribution
3010 L
Clearance
402 89 mL/min
Following oral administration of Entereg capsules in healthy volunteers, plasma alvimopan concentration peaked at approximately 2 hours postdose. No significant accumulation in alvimopan concentration was observed following twice daily (BID) dosing. The mean peak plasma concentration was 10.98 (+ or - 6.43) ng/mL and mean AUC0-12hr was 40.2 (+ or - 22.5) ng hr/mL after dosing of alvimopan at 12 mg BID for 5 days. The absolute bioavailability was estimated to be 6% (range, 1% to 19%). Plasma concentrations of alvimopan increased approximately proportionally with increasing doses between 6 and 18 mg, but less than proportionally from 18 to 24 mg.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
Alvimopan and its metabolite are distributed into milk in rats; it is unknown whether the drug or its metabolite is distributed into human milk.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 3037
The steady state volume of distribution of alvimopan was estimated to be 30 + or - 10 L. Plasma protein binding of alvimopan and its metabolite was independent of concentration over ranges observed clinically and averaged 80% and 94%, respectively. Both alvimopan and the metabolite were bound to albumin and not to alpha-1 acid glycoprotein.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
The average plasma clearance for alvimopan was 402 (+ or - 89) mL/min. Renal excretion accounted for approximately 35% of total clearance. There was no evidence that hepatic metabolism was a significant route for alvimopan elimination. Biliary secretion was considered the primary pathway for alvimopan elimination. Unabsorbed drug and unchanged alvimopan resulting from biliary excretion were then hydrolyzed to its metabolite by gut microflora. The metabolite was eliminated in the feces and in the urine as unchanged metabolite, the glucuronide conjugate of the metabolite, and other minor metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
... The population pharmacokinetics of orally administered alvimopan and its primary metabolite in healthy subjects/special populations, and surgical patients at risk for ileus /was characterized/. Models were consistent with known physiology/pharmacology. Alvimopan's model had two compartments with first-order elimination. Metabolite was modeled with a catenary chain and lag for alvimopan's metabolism within the gut followed by absorption, one systemic compartment with first-order elimination. Weight, gender, and renal function did not affect alvimopan or metabolite. Steady-state alvimopan and metabolite concentrations were 87 and 40% higher, respectively, in patients. Alvimopan concentrations were 35% higher in the elderly, but were not affected by race, acid blockers, or antibiotics. Metabolite concentrations were 43 and 82% lower in African Americans and Hispanics, respectively, compared to Caucasians, 49% lower with acid blockers and 81% lower with preoperative antibiotics. Although alvimopan's pharmacokinetics was described with a traditional model, its metabolite required a novel model accommodating gut metabolism.
PMID:17653140 Foss JF et al; Clin Pharmacol Ther 83 (5): 770-6 (2008)
Alvimopan is primarily metabolized by intestinal flora to an active metabolite although it has no clinically significant contribution to the effects of the drug.
... There was no evidence that hepatic metabolism was a significant route for alvimopan elimination. ... Unabsorbed drug and unchanged alvimopan resulting from biliary excretion were then hydrolyzed to its metabolite by gut microflora. The metabolite was eliminated in the feces and in the urine as unchanged metabolite, the glucuronide conjugate of the metabolite, and other minor metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
Following oral administration of alvimopan, an amide hydrolysis compound is present in the systemic circulation, which is considered a product exclusively of intestinal flora metabolism. This compound is referred to as the metabolite. It is also a mu-opioid receptor antagonist with a Ki of 0.8 nM (0.3 ng/mL). ... There was a delay in the appearance of the metabolite, which had a median Tmax of 36 hours following administration of a single dose of alvimopan. Concentrations of the metabolite were highly variable between subjects and within a subject. The metabolite accumulated after multiple doses of ENTEREG. The mean Cmax for the metabolite after alvimopan 12 mg twice daily for 5 days was 35.73 + or - 35.29 ng/mL.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
10 to 17 hours (gut metabolite: 10 to 18 hours)
The mean terminal phase half-life of alvimopan after multiple oral doses of Entereg ranged from 10 to 17 hours. The terminal half-life of the metabolite ranged 10 to 18 hours.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
Alvimopan competitively binds to mu-opioid receptor in the gastrointestinal tract but, unlike methylnaltrexone which relies upon it ionic charge, alvimopan owes its selectivity for peripheral receptors to its pharmacokinetics. Alvimopan binds to peripheral mu-receptors with a Ki of 0.2 ng/mL.
Alvimopan is a selective antagonist of the cloned human mu-opioid receptor with a Ki of 0.4 nM (0.2 ng/mL) and no measurable opioid-agonist effects in standard pharmacologic assays. The dissociation of (3)H-alvimopan from the human mu-opioid receptor is slower than that of other opioid ligands, consistent with its higher affinity for the receptor. At concentrations of 1 to 10 uM, alvimopan demonstrated no activity at any of over 70 non-opioid receptors, enzymes, and ion channels.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
Following oral administration, alvimopan antagonizes the peripheral effects of opioids on gastrointestinal motility and secretion by competitively binding to gastrointestinal tract mu-opioid receptors. The antagonism produced by alvimopan at opioid receptors is evident in isolated guinea pig ileum preparations where alvimopan competitively antagonizes the effects of morphine on contractility. Alvimopan achieves this selective gastrointestinal opioid antagonism without reversing the central analgesic effects of mu-opioid agonists.
US Natl Inst Health; DailyMed. Current Medication Information for ENTEREG (alvimopan) capsule (July 2011). Available from, as of February 4, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=826f647e-1c29-4f27-2db7-89d4d0f5f105
This study characterized the pharmacology of the peripherally restricted opioid receptor antagonists, alvimopan, its metabolite, ADL 08-0011, and methylnaltrexone. The activities of the compounds were investigated with respect to human or guinea pig opioid receptor binding and function in recombinant cell lines and mechanical responsiveness of the guinea pig ileum. Alvimopan and ADL 08-0011 had higher binding affinity than methylnaltrexone at human mu opioid receptors (pK (i) values of 9.6, 9.6, and 8.0, respectively). The compounds had different selectivities for the mu receptor over human delta and guinea pig kappa opioid receptors. ADL 08-0011 had the highest mu receptor selectivity. With respect to their mu opioid receptor functional activity ([(35)S]GTPgammaS incorporation), methylnaltrexone had a positive intrinsic activity, consistent with partial agonism, unlike alvimopan and ADL 08-0011, which had negative intrinsic activities. Alvimopan, ADL 08-0011, and methylnaltrexone antagonized inhibitory responses mediated by the mu opioid agonist, endomorphin-1 (pA (2) values of 9.6, 9.4, and 7.6, respectively) and by U69593, a kappa opioid agonist (pA (2) values of 8.4, 7.2, and 6.7, respectively). In morphine-naive guinea pig ileum, methylnaltrexone reduced, while alvimopan and ADL 08-0011 increased, the amplitude of electrically evoked contractions and spontaneous mechanical activity. In tissue from morphine-dependent animals, alvimopan and ADL 08-0011 increased spontaneous activity to a greater degree than methylnaltrexone. The data suggested that alvimopan-induced contractions resulted predominantly from an interaction with kappa opioid receptors. It is concluded that alvimopan, ADL 08-0011, and methylnaltrexone differ in their in vitro pharmacological properties, particularly with respect to opioid receptor subtype selectivity and intrinsic activity.
PMID:17340127 Beattie DT et al; Naunyn Schmiedebergs Arch Pharmacol 375 (3): 205-20 (2007)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
92
PharmaCompass offers a list of Alvimopan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Alvimopan manufacturer or Alvimopan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Alvimopan manufacturer or Alvimopan supplier.
PharmaCompass also assists you with knowing the Alvimopan API Price utilized in the formulation of products. Alvimopan API Price is not always fixed or binding as the Alvimopan Price is obtained through a variety of data sources. The Alvimopan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Alvimopan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Alvimopan, including repackagers and relabelers. The FDA regulates Alvimopan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Alvimopan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Alvimopan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Alvimopan supplier is an individual or a company that provides Alvimopan active pharmaceutical ingredient (API) or Alvimopan finished formulations upon request. The Alvimopan suppliers may include Alvimopan API manufacturers, exporters, distributors and traders.
click here to find a list of Alvimopan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Alvimopan DMF (Drug Master File) is a document detailing the whole manufacturing process of Alvimopan active pharmaceutical ingredient (API) in detail. Different forms of Alvimopan DMFs exist exist since differing nations have different regulations, such as Alvimopan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Alvimopan DMF submitted to regulatory agencies in the US is known as a USDMF. Alvimopan USDMF includes data on Alvimopan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Alvimopan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Alvimopan suppliers with USDMF on PharmaCompass.
A Alvimopan written confirmation (Alvimopan WC) is an official document issued by a regulatory agency to a Alvimopan manufacturer, verifying that the manufacturing facility of a Alvimopan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Alvimopan APIs or Alvimopan finished pharmaceutical products to another nation, regulatory agencies frequently require a Alvimopan WC (written confirmation) as part of the regulatory process.
click here to find a list of Alvimopan suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Alvimopan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Alvimopan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Alvimopan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Alvimopan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Alvimopan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Alvimopan suppliers with NDC on PharmaCompass.
Alvimopan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Alvimopan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Alvimopan GMP manufacturer or Alvimopan GMP API supplier for your needs.
A Alvimopan CoA (Certificate of Analysis) is a formal document that attests to Alvimopan's compliance with Alvimopan specifications and serves as a tool for batch-level quality control.
Alvimopan CoA mostly includes findings from lab analyses of a specific batch. For each Alvimopan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Alvimopan may be tested according to a variety of international standards, such as European Pharmacopoeia (Alvimopan EP), Alvimopan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Alvimopan USP).

