
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
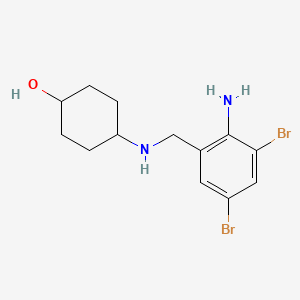

1. 4-(((2-amino-3,5-dibromophenyl)methyl)amino)cyclohexanol
2. Abrohexal
3. Am, Bisolvon
4. Ambril
5. Ambro Puren
6. Ambro-puren
7. Ambrobeta
8. Ambrofur
9. Ambrohexal
10. Ambrols
11. Ambrolitic
12. Ambropp
13. Ambropuren
14. Ambroten
15. Ambroxin
16. Ambroxocompren
17. Bisolvon Am
18. Bromhexine Metabolite Viii
19. Bronchopront
20. Bronchowern
21. Broxol
22. Contac Husten Trunk
23. Contac Husten-trunk
24. Contac Hustentrunk
25. Dinobroxol
26. Duramucal
27. Ebromin
28. Expeflen
29. Expit
30. Farmabroxol
31. Flavamed
32. Frenopect
33. Gelopol
34. Hustenlser, Pect
35. Hustenlser, Therapin
36. Larylin Husten Lser
37. Larylin Husten-lser
38. Larylin Hustenlser
39. Lasolvan
40. Metabolite Viii, Bromhexine
41. Mibrox
42. Motosol
43. Muco Fips
44. Muco-fips
45. Mucofips
46. Mucosolvan
47. Mucotablin
48. Na 872
49. Na-872
50. Na872
51. Pdiamuc
52. Pect Hustenlser
53. Pulmonal S
54. Pulmonal, Ringelheimer
55. Ringelheimer Pulmonal
56. Sekretovit
57. Stas Hustenlser
58. Stas-hustenlser
59. Stashustenlser
60. Surbronc
61. Therapin Hustenlser
1. 18683-91-5
2. 107814-37-9
3. Rac-cis-ambroxol
4. 36557-04-7
5. Ambroxol Base
6. 4-((2-amino-3,5-dibromobenzyl)amino)cyclohexan-1-ol
7. Ambroxolum
8. Bisolvon Metabolite Viii
9. 4-[(2-amino-3,5-dibromobenzyl)amino]cyclohexanol
10. Bromhexine-metabolite Viii
11. Na-872
12. Tabcin
13. Trans-4-((2-amino-3,5-dibromobenzyl)amino)cyclohexanol
14. Ambroxol, Cis-
15. Cis-4-((2-amino-3,5-dibromobenzyl)amino)cyclohexanol
16. Ambroxol [inn]
17. Cyclohexanol, 4-[[(2-amino-3,5-dibromophenyl)methyl]amino]-, Trans-
18. 4-[(2-amino-3,5-dibromophenyl)methylamino]cyclohexan-1-ol
19. Trans-4-((2-amino-3,5-dibromobencil)amino)ciclohexanol
20. Trans-4-((2-amino-3,5-dibromobenzyl)amine)cyclohexanol
21. Trans-4-[(2-amino-3,5-dibromobenzyl)amino]cyclohexanol
22. Qh6zt6j071
23. Cyclohexanol, 4-((2-amino-3,5-dibromobenzyl)amino)- (e)-
24. N-(2-amino-3,4-dibromocyclohexyl)-trans-4-aminocyclohexanol
25. 4-hydroxydemethylbromhexine, Cis-
26. N-(trans-4-hydroxycyclohexyl)-(2-amino-3,5-dibromobenzyl)-amine
27. N-(trans-p-hydroxycyclohexyl)-(2-amino-3,5-dibromobenzyl)amine
28. Ambroxol (inn)
29. Cyclohexanol, 4-(((2-amino-3,5-dibromophenyl)methyl)amino)-, Trans-
30. 200168s0cl
31. N-(2-amino-3,4-dibromociclohexil)-trans-4-aminociclohexanol
32. N-(trans-4-hidroxiciclohexil)-(2-amino-3,5-dibromobencil)amina
33. Ambroxol [inn:ban]
34. Cis-4-(((2-amino-3,5-dibromophenyl)methyl)amino)cyclohexanol
35. Ambroxolum [inn-latin]
36. Cyclohexanol, 4-(((2-amino-3,5-dibromophenyl)methyl)amino)-, Cis-
37. Cyclohexanol, 4-(((2-amino-3,5-dibromophenyl)methyl)amino)-, Trans- (9ci)
38. Bromhexine Metabolite Viii
39. Ncgc00016781-04
40. Einecs 242-500-3
41. Cas-23828-92-4
42. Amboxol
43. Cis-ambroxol
44. Sr-05000001463
45. Unii-200168s0cl
46. Tabcin (tn)
47. Spectrum_001346
48. 4-{[(2-amino-3,5-dibromophenyl)methyl]amino}cyclohexan-1-ol
49. Ambroxol [mi]
50. Rac-cis-ambroxol-[d5]
51. Prestwick0_000366
52. Prestwick1_000366
53. Prestwick2_000366
54. Prestwick3_000366
55. Spectrum2_001518
56. Spectrum3_000955
57. Spectrum4_001068
58. Spectrum5_001021
59. Trans-4-((2-amino-3,5-dibromobencil)amino)ciclohexanol [spanish]
60. N-(2-amino-3,4-dibromociclohexil)-trans-4-aminociclohexanol [spanish]
61. Ambroxol [who-dd]
62. Ec 242-500-3
63. N-(trans-4-hidroxiciclohexil)-(2-amino-3,5-dibromobencil)amina [spanish]
64. Unii-qh6zt6j071
65. Oprea1_766685
66. Schembl18702
67. Bspbio_000491
68. Kbiogr_001396
69. Kbioss_001826
70. Mls001306470
71. Divk1c_000027
72. Schembl423712
73. Spbio_001595
74. Spbio_002412
75. Bpbio1_000541
76. Chembl153479
77. Schembl7855003
78. 4-[(2-amino-3,5-dibromo-phenyl)methylamino]cyclohexanol
79. Chembl1477775
80. Dtxsid8022583
81. Schembl21765132
82. Bcbcmap01_000092
83. Chebi:92994
84. Gtpl10692
85. Kbio1_000027
86. Kbio2_001826
87. Kbio2_004394
88. Kbio2_006962
89. Kbio3_002050
90. Dtxsid60860228
91. Chebi:135590
92. Ninds_000027
93. Cyclohexanol, 4-[[(2-amino-3,5-dibromophenyl)methyl]amino]-
94. Hms2089d06
95. Hms2231b19
96. Hms3373j22
97. Hms3886m18
98. Zinc587613
99. Albb-022461
100. Bcp04489
101. Hy-b1039
102. Bbl009896
103. Bdbm50395322
104. Mfcd00242702
105. Mfcd28143339
106. S5710
107. Stk711075
108. Akos005530704
109. Akos015889660
110. Akos027338704
111. Zinc100001905
112. Zinc100070274
113. Ac-8362
114. Ambroxol Hydrochloride Impurity D [ep]
115. Bcp9000283
116. Ccg-207907
117. Cs-4558
118. Db06742
119. Sb17463
120. Sb82866
121. Idi1_000027
122. Smp1_000014
123. Ncgc00016781-01
124. Ncgc00016781-02
125. Ncgc00016781-08
126. Ncgc00159399-02
127. Ncgc00371077-02
128. As-56023
129. Smr000718792
130. Vs-02240
131. 4-hydroxydemethylbromhexine, Trans-
132. Bcp0726000066
133. Sbi-0051766.p002
134. Ab00514663
135. Cs-0323348
136. Ft-0622261
137. Ft-0630430
138. Ft-0661549
139. D07442
140. 4-(2-amino-3,5-dibromobenzylamino)cyclohexanol
141. Ab00053639_13
142. Ab01275465-01
143. 683a915
144. A813089
145. Q221637
146. Sr-05000001463-1
147. Ambroxol Hydrochloride Impurity D [ep Impurity]
148. Brd-k11223672-003-03-7
149. Brd-k11223672-003-04-5
150. Brd-k56558538-003-02-8
151. 4-[(2-amino-3,5-dibromophenyl)methylamino-]cyclohexanol
152. 4-[(2-amino-3,5-dibromophenyl)methylamino]cyclohexanol
153. (1s,4s)-4-((2-amino-3,5-dibromobenzyl)amino)cyclohexanol
154. 4-[(2-amino-3,5-dibromobenzyl)amino]cyclohexanol, Trans-
155. (1r,4r)-4-((2-amino-3,5-dibromobenzyl)amino)cyclohexan-1-ol
156. Trans-4-{[(2-amino-3,5-dibromophenyl)methyl]amino}cyclohexanol
157. Cyclohexanol, 4-[[(2-amino-3,5-dibromo-phenyl)methyl]amino]-, Trans
| Molecular Weight | 378.10 g/mol |
|---|---|
| Molecular Formula | C13H18Br2N2O |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 377.97654 g/mol |
| Monoisotopic Mass | 375.97859 g/mol |
| Topological Polar Surface Area | 58.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 259 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ambroxol is indicated for secretolytic therapy in bronchoplmonary disease with abnormal mucus secretion and transport. It allows the mucus to be more easily cleared and ease a patient's breathing.
Expectorants
Agents that increase mucous excretion. Mucolytic agents, that is drugs that liquefy mucous secretions, are also included here. (See all compounds classified as Expectorants.)
R05CB06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R05 - Cough and cold preparations
R05C - Expectorants, excl. combinations with cough suppressants
R05CB - Mucolytics
R05CB06 - Ambroxol
Absorption
Rapid and almost complete.
Ambroxol has known human metabolites that include 2-Amino-3,5-dibromobenzaldehyde and 4-Aminocyclohexanol, cis-.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
7-12 hours
Ambroxol is a mucolytic agent. Excessive Nitric oxide (NO) is associated with inflammatory and some other disturbances of airways function. NO enhances the activation of soluble guanylate cyclase and cGMP accumulation. Ambroxol has been shown to inhibit the NO-dependent activation of soluble guanylate cyclase. It is also possible that the inhibition of NO-dependent activation of soluble guanylate cyclase can suppress the excessive mucus secretion, therefore it lowers the phlegm viscosity and improves the mucociliary transport of bronchial secretions.









 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
76
PharmaCompass offers a list of Ambroxol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ambroxol manufacturer or Ambroxol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ambroxol manufacturer or Ambroxol supplier.
PharmaCompass also assists you with knowing the Ambroxol API Price utilized in the formulation of products. Ambroxol API Price is not always fixed or binding as the Ambroxol Price is obtained through a variety of data sources. The Ambroxol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ambroxol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ambroxol, including repackagers and relabelers. The FDA regulates Ambroxol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ambroxol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ambroxol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ambroxol supplier is an individual or a company that provides Ambroxol active pharmaceutical ingredient (API) or Ambroxol finished formulations upon request. The Ambroxol suppliers may include Ambroxol API manufacturers, exporters, distributors and traders.
click here to find a list of Ambroxol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ambroxol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ambroxol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ambroxol GMP manufacturer or Ambroxol GMP API supplier for your needs.
A Ambroxol CoA (Certificate of Analysis) is a formal document that attests to Ambroxol's compliance with Ambroxol specifications and serves as a tool for batch-level quality control.
Ambroxol CoA mostly includes findings from lab analyses of a specific batch. For each Ambroxol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ambroxol may be tested according to a variety of international standards, such as European Pharmacopoeia (Ambroxol EP), Ambroxol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ambroxol USP).
