
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. A.m.k
2. Amikacin Sulfate
3. Amikacina Medical
4. Amikacina Normon
5. Amikafur
6. Amikalem
7. Amikason's
8. Amikayect
9. Amikin
10. Amiklin
11. Amukin
12. Bb K 8
13. Bb K8
14. Bb-k 8
15. Bb-k8
16. Bbk 8
17. Bbk8
18. Biclin
19. Biklin
20. Gamikal
21. Kanbine
22. Medical, Amikacina
23. Normon, Amikacina
24. Oprad
25. Sulfate, Amikacin
26. Yectamid
1. 37517-28-5
2. Amicacin
3. Amikin
4. Amikacinum
5. Lukadin
6. Amikacine
7. Amikacina
8. Amikacillin
9. Amikacin Sulfate
10. Antibiotic Bb-k 8
11. Potentox
12. Amukin
13. Amikacine [inn-french]
14. Amikacinum [inn-latin]
15. Amikacina [inn-spanish]
16. Amikacin Base
17. Bb-k8
18. Kaminax
19. 1-n-(l(-)-gamma-amino-alpha-hydroxybutyryl)kanamycin A
20. Bay 41-6551
21. Amikacin Free Base
22. Briclin
23. Amikavet
24. Chebi:2637
25. Amiglyde-v
26. Arikace
27. N1-[(s)-4-amino-2-hydroxybutyryl]kanamycin A
28. Antibiotic Bb-k8
29. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-[(2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxyoxan-2-yl]oxy-3-hydroxycyclohexyl]-2-hydroxybutanamide
30. (2s)-4-amino-n-{(1r,2s,3s,4r,5s)-5-amino-2-[(3-amino-3-deoxy-alpha-d-glucopyranosyl)oxy]-4-[(6-amino-6-deoxy-alpha-d-glucopyranosyl)oxy]-3-hydroxycyclohexyl}-2-hydroxybutanamide
31. Mikavir
32. 84319sgc3c
33. 37517-28-5 (free Base)
34. Amikacin Dihydrate
35. Nsc177001
36. Nsc-177001
37. Bb-k 8
38. Ncgc00093350-02
39. Dsstox_cid_2586
40. Dsstox_rid_76645
41. Dsstox_gsid_22586
42. (s)-4-amino-n-((1r,2s,3s,4r,5s)-5-amino-2-(((2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)-4-(((2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxytetrahydro-2h-pyran-2-yl)oxy)-3-hydroxycyclohexyl)-2-hydroxybutanamide
43. Amk
44. Amikozit
45. Hsdb 3583
46. Amikacin (usp/inn)
47. Einecs 253-538-5
48. Nsc 177001
49. Amikacin [usp:inn:ban]
50. Amikacin Inhalation Solution
51. Unii-84319sgc3c
52. Sr-01000722004
53. Arikayce Liposomal
54. Ncgc00093350-05
55. Amikacin,(s)
56. (2s)-n-[(1r,2s,3s,4r,5s)-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5-azanyl-2-[(2s,3r,4s,5s ,6r)-4-azanyl-6-(hydroxymethyl)-3,5-bis(oxidanyl)oxan-2-yl]oxy-3-oxidanyl-cyclohexyl]-4-azanyl-2-oxidanyl-butanamide
57. (2s)-n-[(1r,2s,3s,4r,5s)-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5-azanyl-2-[(2s,3r,4s,5s,6r)-4-azanyl-6-(hydroxymethyl)-3,5-bis(oxidanyl)oxan-2-yl]oxy-3-oxidanyl-cyclohexyl]-4-azanyl-2-oxidanyl-butanamide
58. Akn
59. Amikin (disulfate)
60. Mfcd00883675
61. Bay41-6551
62. Amikacin (free Base)
63. O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1-4)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1-6))-n(sup 3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine
64. Nktr-061
65. Amikacin [hsdb]
66. Amikacin [inn]
67. Amikacin [jan]
68. Amikacin [mi]
69. Amikacin [vandf]
70. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-[(2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxyoxan-2-yl]oxy-3-hydroxycyclohexyl]
71. Prestwick3_000395
72. Amikacin [mart.]
73. Amikacin [usp-rs]
74. Amikacin [who-dd]
75. Amikacin [who-ip]
76. Chembl177
77. Schembl2985
78. Cas-37517-28-5
79. Bspbio_000609
80. D-streptamine, O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1-6)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1-4))-n1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-
81. Bpbio1_000671
82. Amikacin [ep Monograph]
83. Amikacin [usp Monograph]
84. Dtxsid3022586
85. 37517-28-5 (anhydr
86. Gtpl10894
87. Hy-b0509a
88. Mat2501
89. Amikacinum [who-ip Latin]
90. Zinc8214483
91. Tox21_111201
92. Bdbm50237603
93. Stk039706
94. Akos005383276
95. Tox21_111201_1
96. Bay 416651
97. Db00479
98. Ncgc00093350-03
99. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-(3-amino-3-deoxy-alpha-d-glucopyranosyloxy)-4-(6-amino-6-deoxy-alpha-d-glucopyranosyloxy)-3-hydroxycyclohexyl]-2-hydroxybutanamide
100. As-76985
101. D-streptamine, O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1->6)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1->4))-n(sup 1)-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-
102. D-streptamine, O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1-6)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1-4))-n(sup 1)-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-
103. O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->6)-o-[6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->4)]-1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-d-streptamine
104. O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1->4)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1->6))-n(3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine
105. Ab00513828
106. C06820
107. D02543
108. Ab00513828_06
109. Amikacin, Antibiotic For Culture Media Use Only
110. 517a285
111. A823716
112. Q408529
113. Sr-01000722004-6
114. W-106531
115. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-[(2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxy-tetrahydropyran-2-yl]oxy-3-hydroxy-cyclohexyl]-2-hydroxy-butanamide
116. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-{[(2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4-{[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-3-hydroxycyclohexyl]-2-hydroxybutanamide
117. (2s)-n-[(1r,2s,3s,4r,5s)-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5-azanyl-2-[(2s,3r,4s,5s
118. (s)-4-amino-n-((1r,2s,3s,4r,5s)-5-amino-2-((2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yloxy)-4-((2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxytetrahydro-2h-pyran-2-yloxy)-3-hydroxycyclohexyl)-2-hydroxybutanamide
119. D-streptamine, O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->6)-o-(6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->4))-n(sup 1)-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-
120. D-streptamine, O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->6)-o-[6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->4)]-n1-[(2s)-4-amino-2-hydroxy-1-oxobutyl]-2-deoxy-
121. D-streptamine, O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1.fwdarw.6)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1.fwdarw.4))-n1-((2s)-4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-
122. O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->4)-o-(6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->6))-n(sup 3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine
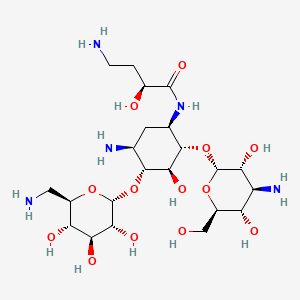
| Molecular Weight | 585.6 g/mol |
|---|---|
| Molecular Formula | C22H43N5O13 |
| XLogP3 | -7.9 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 10 |
| Exact Mass | 585.28573644 g/mol |
| Monoisotopic Mass | 585.28573644 g/mol |
| Topological Polar Surface Area | 332 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 819 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 16 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Amikacin sulfate |
| Drug Label | Amikacin Sulfate Injection USP is a semi-synthetic aminoglycoside antibiotic derived from kanamycin. D-Streptamine,O-3-amino-3-deoxy--D-glucopyranosyl-(16)-O-[6-amino-6-deoxy--D-glucopyranosyl-(14)]-N1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy... |
| Active Ingredient | Amikacin sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 250mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms; Teva Pharms Usa; Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Amikacin sulfate |
| Drug Label | Amikacin Sulfate Injection USP is a semi-synthetic aminoglycoside antibiotic derived from kanamycin. D-Streptamine,O-3-amino-3-deoxy--D-glucopyranosyl-(16)-O-[6-amino-6-deoxy--D-glucopyranosyl-(14)]-N1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy... |
| Active Ingredient | Amikacin sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 250mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms; Teva Pharms Usa; Eurohlth Intl |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings. Amikacin. Online file (MeSH, 2016). Available from, as of December 5, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Amikacin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=AMIKACIN&Search=Search
Amikacin is used for the short-term treatment of serious infections caused by susceptible gram-negative bacteria, including Acinetobacter, Escherichia coli, Enterobacter, Klebsiella, Proteus, Providencia, Pseudomonas, or Serratia marcescens. Amikacin may be the preferred aminoglycoside for initial treatment of serious nosocomial gram-negative infections, especially in areas where resistance to gentamicin and tobramycin has been reported. /Included in US product labeling/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 32
Amikacin is used for the treatment of serious intra-abdominal infections (including peritonitis) caused by susceptible gram-negative bacteria, including Acinetobacter, Enterobacter, E. coli, Klebsiella, Proteus, Providencia, Serratia, or Pseudomonas. Amikacin usually is used as an adjunct to other appropriate anti-infectives (e.g., clindamycin, metronidazole, piperacillin and tazobactam, ampicillin and sulbactam). /Included in US product labeling/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 32
For more Therapeutic Uses (Complete) data for Amikacin (15 total), please visit the HSDB record page.
/BOXED WARNING/ WARNINGS: Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established. Neurotoxicity, manifested as vestibular and permanent bilateral auditory ototoxicity, can occur in patients with preexisting renal damage and in patients with normal renal function treated at higher doses and/or for periods longer than those recommended. The risk of aminoglycoside-induced ototoxicity is greater in patients with renal damage. High frequency deafness usually occurs first and can be detected only by audiometric testing. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions. The risk of hearing loss due to aminoglycosides increases with the degree of exposure to either high peak or high trough serum concentrations. Patients developing cochlear damage may not have symptoms during therapy to warn them of developing eighth-nerve toxicity, and total or partial irreversible bilateral deafness may occur after the drug has been discontinued. Aminoglycoside-induced ototoxicity is usually irreversible.Aminoglycosides are potentially nephrotoxic. The risk of nephrotoxicity is greater in patients with impaired renal function and in those who receive high doses or prolonged therapy. Neuromuscular blockade and respiratory paralysis have been reported following parenteral injection, topical instillation (as in orthopedic and abdominal irrigation or in local treatment of empyema), and following oral use of aminoglycosides. The possibility of these phenomena should be considered if aminoglycosides are administered by any route, especially in patients receiving anesthetics, neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium, or in patients receiving massive transfusions of citrate-anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena, but mechanical respiratory assistance may be necessary. Renal and eighth-nerve function should be closely monitored especially in patients with known or suspected renal impairment at the onset of therapy and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of amikacin should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels and prolonged peak concentrations above 35 micrograms per mL. Urine should be examined for decreased specific gravity, increased excretion of proteins, and the presence of cells or casts. Blood urea nitrogen, serum creatinine, or creatinine clearance should be measured periodically. Serial audiograms should be obtained where feasible in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears, and hearing loss) or nephrotoxicity requires discontinuation of the drug or dosage adjustment. Concurrent and/or sequential systemic, oral or topical use of other neurotoxic or nephrotoxic products, particularly bacitracin, cisplatin, amphotericin B, cephaloridine, paromomycin, viomycin, polymyxin B, colistin, vancomycin, or other aminoglycosides should be avoided. Other factors that may increase risk of toxicity are advanced age and dehydration. The concurrent use of amikacin with potent diuretics (ethacrynic acid, or furosemide) should be avoided since diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue.
NIH; DailyMed. Current Medication Information for Amikacin Sulfate (Amikacin Sulfate Injection, Solution) (Updated: October 2015). Available from, as of February 21, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a43188fa-f228-4acd-8a5d-9a3462034f4b
Toxic effects on the eighth cranial nerve can result in hearing loss, loss of balance, or both. Amikacin primarily affects auditory function. Cochlear damage includes high frequency deafness and usually occurs before clinical hearing loss can be detected.
NIH; DailyMed. Current Medication Information for Amikacin Sulfate (Amikacin Sulfate Injection, Solution) (Updated: October 2015). Available from, as of February 21, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a43188fa-f228-4acd-8a5d-9a3462034f4b
A history of hypersensitivity to amikacin is a contraindication for its use. A history of hypersensitivity or serious toxic reactions to aminoglycosides may contraindicate the use of any other aminoglycoside because of the known cross-sensitivities of patients to drugs in this class.
NIH; DailyMed. Current Medication Information for Amikacin Sulfate (Amikacin Sulfate Injection, Solution) (Updated: October 2015). Available from, as of February 21, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a43188fa-f228-4acd-8a5d-9a3462034f4b
Aminoglycosides should be used with caution in premature and neonatal infants because of the renal immaturity of these patients and the resulting prolongation of serum half-life of these drugs. /Aminoglycosides/
NIH; DailyMed. Current Medication Information for Amikacin Sulfate (Amikacin Sulfate Injection, Solution) (Updated: October 2015). Available from, as of February 21, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a43188fa-f228-4acd-8a5d-9a3462034f4b
For more Drug Warnings (Complete) data for Amikacin (37 total), please visit the HSDB record page.
The amikacin sulfate injection is indicated in the short-term treatment of serious bacterial infections due to susceptible strains of gram-negative bacteria, including Pseudomonas species, Escherichia coli, species of indole-positive and indole-negative Proteus, Providencia species, Klebsiella-Enterobacter-Serratia species, as well as Acinetobacter (Mima-Herellea) species. Clinical studies have shown amikacin sulfate injection to be effective in bacterial septicemia (including neonatal sepsis); in serious infections of the respiratory tract, bones and joints, central nervous system (including meningitis) and skin and soft tissue; intra-abdominal infections (including peritonitis); and in burns and postoperative infections (including post-vascular surgery). Clinical studies have shown amikacin also to be effective in serious, complicated, and recurrent urinary tract infections due to the above organisms. Aminoglycosides, including amikacin, are not indicated in uncomplicated first-time episodes of urinary tract infections unless the causative organisms are not susceptible to antibiotics which are less toxic. In September 2018, a new indication with a new dosage route was approved for this drug. Amikacin liposome inhalation suspension was approved for the treatment of lung disease caused by a group of bacteria, Mycobacterium avium complex (MAC) in a limited population of patients with the disease who do not respond to conventional treatment (refractory disease). This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6 of treatment. Clinical benefit has not yet been established. **Important notes regarding Staphylococcus and Sensitivity testing:** Staphylococcus aureus, including methicillin-resistant strains, is the principal Gram-positive organism sensitive to amikacin. The use of amikacin in the treatment of staphylococcal infections should be restricted only to second-line therapy, and should be limited to only those patients suffering from severe infections caused by susceptible strains of staphylococcus species who have failed to show sensitivity to other available antibiotics. Bacteriologic studies should be performed to identify causative organisms and their susceptibilities to amikacin. Amikacin may be used as initial therapy in suspected gram-negative infections and therapy may be initiated before obtaining the results of susceptibility testing.
FDA Label
Arikayce liposomal is indicated for the treatment of non-tuberculous mycobacterial (NTM) lung infections caused by Mycobacterium avium Complex (MAC) in adults with limited treatment options who do not have cystic fibrosis.
Amikacin is an aminoglycoside antibiotic. Aminoglycosides bind to the bacteria, causing misreading of t-RNA, leaving bacteria unable to synthesize proteins vital to their growth. Aminoglycosides are useful mainly in the treatment infections involving aerobic, Gram-negative bacteria, such as Pseudomonas, Acinetobacter, and Enterobacter. In addition, some mycobacteria, including the bacteria that cause tuberculosis, are susceptible to aminoglycosides. Infections caused by Gram-positive bacteria can also be treated with aminoglycosides, however, other antibiotics may be more potent and less toxic to humans.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01GB06
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX12 - Amikacin
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01G - Aminoglycoside antibacterials
J01GB - Other aminoglycosides
J01GB06 - Amikacin
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA21 - Amikacin
Absorption
Rapidly absorbed after intramuscular administration. Rapid absorption occurs from the peritoneum and pleura. Poor oral and topical absorption. Poorly absorbed from bladder irrigations and intrathecal administration. The bioavailability of this drug is expected to vary primarily from individual differences in nebulizer efficiency and airway pathology. Following IM administration of a single dose of amikacin of 7.5 mg/kg in adults with normal renal function, peak plasma amikacin concentrations of 17-25 micrograms/mL are attained within 45 minutes to 2 hours. Following IV infusion of the same dose given over 1 hour peak plasma concentrations of the drug average 38 micrograms/mL immediately following the infusion, 5.5 micrograms/mL at 4 hours, and 1.3 micrograms/mL at 8 hours.
Route of Elimination
This drug is eliminated by the kidneys. In adults with normal renal function, 94-98% of a single IM or IV dose of amikacin is excreted unchanged by glomerular filtration in the kidney within 24 hours. Amikacin can be completely recovered within approximately 10-20 days in patients with normal, healthy renal function. In patients with impaired renal function, the clearance of amikacin is found to be decreased; the more severe the impairment, the slower the clearance. The interval between doses of amikacin should be adjusted according to the level of renal impairment. Endogenous creatinine clearance rate and serum creatinine which have a high correlation with serum half-life of amikacin, may be used as a guide for dosing.
Volume of Distribution
24 L (28% of body weight healthy adult subjects). Following administration of usual dosages of amikacin, amikacin has been found in bone, heart, gallbladder, and lung tissue. Amikacin is also distributed into bile, sputum, bronchial secretions, and interstitial, pleural, and synovial fluids.
Clearance
The mean serum clearance rate is about 100 mL/min and the renal clearance rate is 94 mL/min in subjects with normal renal function.
Emergence of a multiply drug resistant Enterobacter cloacae during a seven-week period in 1980 caused amikacin to become the aminoglycoside of choice in the initial management of suspected sepsis in a neonatal intensive care unit. Recommended doses (7.5-10 mg/kg loading; 15 mg/kg in two divided doses IV) were given to 5 infants < or = 1,000 gm and to 13 larger babies. Trough levels 11.5 hours after a dose were 16.6 +/- 11.9 ug/mL in infants < or = 1,000 gm and 6.5 +/- 4.3 ug/mL in the larger infants (P < 0.02). Peak levels one hour postinfusion exceeded 40 ug/mL in 3 of 5 < or = 1,000-gm babies and 4 of 12 > 1,000-gm infants (P = NS). Overall, 7 of 10 peak and/or trough levels in < or = 1,000-gm infants were in the range considered toxic in adults, versus 7 of 24 in larger babies (P = 0.03). These data show that ... excessive blood levels of amikacin are likely in infants < or = 1,000 gm and may also occur in larger infants using currently recommended dosage schedules. These ... findings emphasize the need to monitor drug levels and individualize therapy in very low birthweight infants.
PMID:12760404 Philips JB 3rd et al; Pediatr Pharmacol (New York) 2 (2): 121-5 (1982)
Amikacin is poorly absorbed from the GI tract. Amikacin is rapidly absorbed following IM administration. Following IM administration of a single 7.5-mg/kg dose of amikacin in adults with normal renal function, peak plasma amikacin concentrations are attained within about 0.5-2 hours and average 17-25 ug/mL; plasma concentrations 10 hours after the dose average 2.1 ug/mL.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 34
When a 7.5-mg/kg dose of amikacin is administered by IV infusion over 30 minutes, peak plasma concentrations of the drug average 38 ug/mL immediately following the infusion, 18 ug/mL at 1 hour, and 0.75 ug/mL at 10 hours. In adults receiving 15 mg/kg once daily by IV infusion over 30 minutes, peak serum concentrations (measured 30 minutes after completion of an infusion) were 40.9 ug/mL and trough concentrations (measured immediately before start of an infusion) were 1.8 ug/mL.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 34
Accumulation of amikacin does not appear to occur in adult or pediatric patients with normal renal function receiving usual dosages of the drug twice daily for 4-10 days.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 34
For more Absorption, Distribution and Excretion (Complete) data for Amikacin (15 total), please visit the HSDB record page.
Amikacin's structure has been altered to reduce the possible route of enzymatic deactivation, thus reducing bacterial resistance. Many strains of Gram-negative organisms resistant to gentamicin and tobramycin have shown to be sensitive to amikacin in vitro.
Aminoglycosides are not metabolized and are excreted unchanged in the urine primarily by glomerular filtration. /Aminoglycosides/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 31
The plasma elimination half-life of amikacin is usually 2-3 hours in adults with normal renal function and is reported to range from 30-86 hours in adults with severe renal impairment.
The plasma elimination half-life of amikacin usually is 2-3 hours in adults with normal renal function and is reported to range from 28-86 hours in adults with severe renal impairment. The plasma elimination half-life of amikacin is reported to be 4-5 hours in full-term infants 7 days of age or older and 7-8 hours in low birth-weight infants 1-3 days of age. In preterm neonates, half-life is inversely related to postconceptional age and has ranged from 4.5-15.6 hours. In one study in infants and children 20 days to 6 years of age, mean plasma half-life after a single 7.5-mg/kg IM dose was about 2 hours.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 34
The primary mechanism of action of amikacin is the same as that for all aminoglycosides. It binds to bacterial 30S ribosomal subunits and interferes with mRNA binding and tRNA acceptor sites, interfering with bacterial growth. This leads to disruption of normal protein synthesis and production of non-functional or toxic peptides. Other actions have been postulated for drugs of this class. Amikacin, as well as the rest of the aminoglycosides, are generally bacteriocidal against gram-positive and gram-negative bacteria.
Aminoglycosides are usually bactericidal in action. Although the exact mechanism of action has not been fully elucidated, the drugs appear to inhibit protein synthesis in susceptible bacteria by irreversibly binding to 30S ribosomal subunits. /Aminoglycosides/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 29
... Aminoglycosides are aminocyclitols that kill bacteria by inhibiting protein synthesis as they bind to the 16S rRNA and by disrupting the integrity of bacterial cell membrane. Aminoglycoside resistance mechanisms include: (a) the deactivation of aminoglycosides by N-acetylation, adenylylation or O-phosphorylation, (b) the reduction of the intracellular concentration of aminoglycosides by changes in outer membrane permeability, decreased inner membrane transport, active efflux, and drug trapping, (c) the alteration of the 30S ribosomal subunit target by mutation, and (d) methylation of the aminoglycoside binding site. ... /Aminoglycosides/
PMID:17657587 Shakil S et al; J Biomed Sci 15 (1): 5-14 (2008)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
55
PharmaCompass offers a list of Amikacin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amikacin manufacturer or Amikacin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amikacin manufacturer or Amikacin supplier.
PharmaCompass also assists you with knowing the Amikacin API Price utilized in the formulation of products. Amikacin API Price is not always fixed or binding as the Amikacin Price is obtained through a variety of data sources. The Amikacin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amikacin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amikacin, including repackagers and relabelers. The FDA regulates Amikacin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amikacin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amikacin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amikacin supplier is an individual or a company that provides Amikacin active pharmaceutical ingredient (API) or Amikacin finished formulations upon request. The Amikacin suppliers may include Amikacin API manufacturers, exporters, distributors and traders.
click here to find a list of Amikacin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Amikacin DMF (Drug Master File) is a document detailing the whole manufacturing process of Amikacin active pharmaceutical ingredient (API) in detail. Different forms of Amikacin DMFs exist exist since differing nations have different regulations, such as Amikacin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Amikacin DMF submitted to regulatory agencies in the US is known as a USDMF. Amikacin USDMF includes data on Amikacin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Amikacin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Amikacin suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Amikacin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Amikacin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Amikacin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Amikacin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Amikacin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Amikacin suppliers with NDC on PharmaCompass.
Amikacin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amikacin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amikacin GMP manufacturer or Amikacin GMP API supplier for your needs.
A Amikacin CoA (Certificate of Analysis) is a formal document that attests to Amikacin's compliance with Amikacin specifications and serves as a tool for batch-level quality control.
Amikacin CoA mostly includes findings from lab analyses of a specific batch. For each Amikacin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amikacin may be tested according to a variety of international standards, such as European Pharmacopoeia (Amikacin EP), Amikacin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amikacin USP).
