
Synopsis
0
EU WC
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. A.m.k
2. Amikacin
3. Amikacina Medical
4. Amikacina Normon
5. Amikafur
6. Amikalem
7. Amikason's
8. Amikayect
9. Amikin
10. Amiklin
11. Amukin
12. Bb K 8
13. Bb K8
14. Bb-k 8
15. Bb-k8
16. Bbk 8
17. Bbk8
18. Biclin
19. Biklin
20. Gamikal
21. Kanbine
22. Medical, Amikacina
23. Normon, Amikacina
24. Oprad
25. Sulfate, Amikacin
26. Yectamid
1. Amikacin Disulfate
2. 39831-55-5
3. Pierami
4. Amikin
5. Amikacin Bis(sulphate)
6. Kaminax
7. Bb-k8
8. Arikayce
9. Amikacin Sulphate
10. Amikacin Disulphate
11. Amikacin (disulfate)
12. Amikacin (as Sulfate)
13. Amikacin Sulfate Salt
14. Amikacin Disulfate Salt
15. N6m33094fd
16. Nsc-755846
17. Amikafur
18. Amitrex
19. Biodacyn
20. Chemacin
21. Fabianol
22. Likacin
23. Biklin
24. Kancin-gap
25. Antibiotic Bb-k8 Sulfate
26. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-(3-amino-3-deoxy-alpha-d-glucopyranosyloxy)-4-(6-amino-6-deoxy-alpha-d-glucopyranosyloxy)-3-hydroxycyclohexyl]-2-hydroxybutanamide Disulfate
27. Amikacin (sulfate)
28. O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1->4)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1->6))-n(3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine Sulfate (1:2)
29. Nn-k 8
30. Einecs 254-648-6
31. Amikan
32. Unii-n6m33094fd
33. Amikacini Sulfas
34. Amikacin Sulfate [usan:usp:jan]
35. Amikin (tn)
36. Amikin In Sodium Chloride 0.9% In Plastic Container
37. (2s)-4-amino-n-[(1r,2s,3s,4r,5s)-5-amino-2-[(2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-[(2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxyoxan-2-yl]oxy-3-hydroxycyclohexyl]
38. Amikacin Sulfate In Sodium Chloride 0.9% In Plastic Container
39. Bay-416651 Sulfate
40. Amikacin Sulfate [mi]
41. Amikacin Sulfate [jan]
42. Amikacin Sulfate (jp17/usp)
43. Chebi:2638
44. Schembl1649432
45. Amikacin Sulfate [usan]
46. Amikacin Sulfate [vandf]
47. Chembl4208954
48. Hy-b0509b
49. Amikacin Sulfate [mart.]
50. Amikacin Sulfate [usp-rs]
51. Amikacin Sulfate [who-dd]
52. Amikacini Sulfas [who-ip]
53. Dtxsid601045247
54. Mfcd00167475
55. S3065
56. Amikacin Sulfate [green Book]
57. Akos025310105
58. Amikacin Sulfate [ep Impurity]
59. Amikacin Sulfate [orange Book]
60. Amikacin Sulfate [ep Monograph]
61. Ccg-270466
62. Cs-3493
63. Nsc 755846
64. Amikacin Sulfate [usp Monograph]
65. As-13777
66. D-streptamine, O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1-6)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1-4))-n(sup 1)-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-, Sulfate (1:2)(salt)
67. O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1-4)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1-6))-n(sup 3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine Sulfate (1:2)
68. D00865
69. 831a555
70. Q27105754
71. Amikacin Sulfate, European Pharmacopoeia (ep) Reference Standard
72. Amikacin Disulfate Salt, Potency: 674-786 Mug Per Mg (as Amikacin Base)
73. Amikacin Sulfate, United States Pharmacopeia (usp) Reference Standard
74. Amikacin For System Suitability, European Pharmacopoeia (ep) Reference Standard
75. Amikacin Sulfate, Pharmaceutical Secondary Standard; Certified Reference Material
76. (2s)-4-amino-n-{(1r,2s,3s,4r,5s)-5-amino-2-[(3-amino-3-deoxy-alpha-d-glucopyranosyl)oxy]-4-[(6-amino-6-deoxy-alpha-d-glucopyranosyl)oxy]-3-hydroxycyclohexyl}-2-hydroxybutanamide Sulfate (1:2)
77. (s)-4-amino-n-((1r,2s,3s,4r,5s)-5-amino-2-(((2s,3r,4s,5s,6r)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2h-pyran-2-yl)oxy)-4-(((2r,3r,4s,5s,6r)-6-(aminomethyl)-3,4,5-trihydroxytetrahydro-2h-pyran-2-yl)oxy)-3-hydroxycyclohexyl)-2-hydroxybutanamide Bis(sulfate)
78. D-streptamine, O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->6)-o-(6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->4))-n(sup 1)-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-, Sulfate (1:2) (salt)
79. D-streptamine, O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->6)-o-(6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->4))-n(sup 1)-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, (s)-, Sulphate (1:2) (salt)
80. D-streptamine, O-3-amino-3-deoxy-alpha-d-glucopyranosyl-(1.fwdarw.6)-o-(6-amino-6-deoxy-alpha-d-glucopyranosyl-(1.fwdarw.4))-n1-((2s)-4-amino-2-hydroxy-1-oxobutyl)-2-deoxy-, Sulfate (1:2) (salt)
81. O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->4)-o-(6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->6))-n(sup 3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine Sulfate (1:2)
82. O-3-amino-3-deoxy-.alpha.-d-glucopyranosyl-(1->4)-o-(6-amino-6-deoxy-.alpha.-d-glucopyranosyl-(1->6))-n(sup 3)-(4-amino-l-2-hydroxybutyryl)-2-deoxy-l-streptamine Sulphate (1:2)
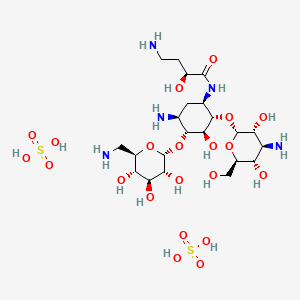
| Molecular Weight | 781.8 g/mol |
|---|---|
| Molecular Formula | C22H47N5O21S2 |
| Hydrogen Bond Donor Count | 17 |
| Hydrogen Bond Acceptor Count | 25 |
| Rotatable Bond Count | 10 |
| Exact Mass | 781.22049587 g/mol |
| Monoisotopic Mass | 781.22049587 g/mol |
| Topological Polar Surface Area | 498 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 900 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 16 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Amikacin sulfate |
| Drug Label | Amikacin Sulfate Injection USP is a semi-synthetic aminoglycoside antibiotic derived from kanamycin. D-Streptamine,O-3-amino-3-deoxy--D-glucopyranosyl-(16)-O-[6-amino-6-deoxy--D-glucopyranosyl-(14)]-N1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy... |
| Active Ingredient | Amikacin sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 250mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms; Teva Pharms Usa; Eurohlth Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Amikacin sulfate |
| Drug Label | Amikacin Sulfate Injection USP is a semi-synthetic aminoglycoside antibiotic derived from kanamycin. D-Streptamine,O-3-amino-3-deoxy--D-glucopyranosyl-(16)-O-[6-amino-6-deoxy--D-glucopyranosyl-(14)]-N1-(4-amino-2-hydroxy-1-oxobutyl)-2-deoxy... |
| Active Ingredient | Amikacin sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 250mg base/ml |
| Market Status | Prescription |
| Company | Emcure Pharms; Teva Pharms Usa; Eurohlth Intl |
Treatment of nontuberculous mycobacterial lung infection, Treatment of Pseudomonas aeruginosa pulmonary infection / colonisation in patients with cystic fibrosis
Arikayce liposomal is indicated for the treatment of non-tuberculous mycobacterial (NTM) lung infections caused by Mycobacterium avium Complex (MAC) in adults with limited treatment options who do not have cystic fibrosis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01GB06

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
76
PharmaCompass offers a list of Amikacin Sulfate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amikacin Sulfate manufacturer or Amikacin Sulfate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amikacin Sulfate manufacturer or Amikacin Sulfate supplier.
PharmaCompass also assists you with knowing the Amikacin Sulfate API Price utilized in the formulation of products. Amikacin Sulfate API Price is not always fixed or binding as the Amikacin Sulfate Price is obtained through a variety of data sources. The Amikacin Sulfate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amikacin Sulfate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amikacin Sulfate, including repackagers and relabelers. The FDA regulates Amikacin Sulfate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amikacin Sulfate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amikacin Sulfate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amikacin Sulfate supplier is an individual or a company that provides Amikacin Sulfate active pharmaceutical ingredient (API) or Amikacin Sulfate finished formulations upon request. The Amikacin Sulfate suppliers may include Amikacin Sulfate API manufacturers, exporters, distributors and traders.
click here to find a list of Amikacin Sulfate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Amikacin Sulfate DMF (Drug Master File) is a document detailing the whole manufacturing process of Amikacin Sulfate active pharmaceutical ingredient (API) in detail. Different forms of Amikacin Sulfate DMFs exist exist since differing nations have different regulations, such as Amikacin Sulfate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Amikacin Sulfate DMF submitted to regulatory agencies in the US is known as a USDMF. Amikacin Sulfate USDMF includes data on Amikacin Sulfate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Amikacin Sulfate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Amikacin Sulfate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Amikacin Sulfate Drug Master File in Japan (Amikacin Sulfate JDMF) empowers Amikacin Sulfate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Amikacin Sulfate JDMF during the approval evaluation for pharmaceutical products. At the time of Amikacin Sulfate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Amikacin Sulfate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Amikacin Sulfate Drug Master File in Korea (Amikacin Sulfate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Amikacin Sulfate. The MFDS reviews the Amikacin Sulfate KDMF as part of the drug registration process and uses the information provided in the Amikacin Sulfate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Amikacin Sulfate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Amikacin Sulfate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Amikacin Sulfate suppliers with KDMF on PharmaCompass.
A Amikacin Sulfate CEP of the European Pharmacopoeia monograph is often referred to as a Amikacin Sulfate Certificate of Suitability (COS). The purpose of a Amikacin Sulfate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Amikacin Sulfate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Amikacin Sulfate to their clients by showing that a Amikacin Sulfate CEP has been issued for it. The manufacturer submits a Amikacin Sulfate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Amikacin Sulfate CEP holder for the record. Additionally, the data presented in the Amikacin Sulfate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Amikacin Sulfate DMF.
A Amikacin Sulfate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Amikacin Sulfate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Amikacin Sulfate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Amikacin Sulfate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Amikacin Sulfate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Amikacin Sulfate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Amikacin Sulfate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Amikacin Sulfate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Amikacin Sulfate suppliers with NDC on PharmaCompass.
Amikacin Sulfate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amikacin Sulfate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amikacin Sulfate GMP manufacturer or Amikacin Sulfate GMP API supplier for your needs.
A Amikacin Sulfate CoA (Certificate of Analysis) is a formal document that attests to Amikacin Sulfate's compliance with Amikacin Sulfate specifications and serves as a tool for batch-level quality control.
Amikacin Sulfate CoA mostly includes findings from lab analyses of a specific batch. For each Amikacin Sulfate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amikacin Sulfate may be tested according to a variety of international standards, such as European Pharmacopoeia (Amikacin Sulfate EP), Amikacin Sulfate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amikacin Sulfate USP).
